Contrast-enhanced Ultrasonography and Color Doppler Ultrasonography for Dynamic Assessment of Intracranial Changes in Early MCAO Rats Models
-
摘要:目的 通过超声造影(contrast-enhanced ultrasound, CEUS)与彩色频谱多普勒超声(color Doppler ultrasound, DUS)探索SD大鼠局灶性永久性缺血性模型早期的血流动力学改变和脑灌注定量参数的变化规律。方法 16只磨薄颅骨后的SD大鼠采用线栓法制备大鼠大脑中动脉闭塞(middle cerebral artery occlusion,MCAO)模型。于造模前(T0)、造模后即刻(造模后5~15 min, T1)、造模后3 h(T2)分别进行脑CEUS成像,并使用DUS测量大鼠双侧大脑中动脉(middle cerebral artery, MCA)、大脑前动脉(anterior cerebral artery, ACA)、大脑后动脉(posterior cerebral artery, PCA)的最大峰值流速(peak systolic velocity, PSV)、舒张末期流速(end-diastolic velocity, EDV)及平均流速(mean velocity, MV)。以右脑整体作为感兴趣区做大脑时间强度曲线,获得CEUS的定量参数:峰值强度(peak intensity, PI)、曲线下面积(area under the curve, AUC)、增强斜率(wash in slope, WIS)、达峰时间(time to peak, TTP)、上升时间(rise time, RT)及峰值下降至一半强度的时间(time from peak to one half, TPH)。造模后3 h行大鼠改良神经功能缺损评分(modified neurological deficit score, mNSS),对评分9~11分的造模成功大鼠的数据进行统计分析。结果 共12只大鼠造模成功。12只大鼠在T1及T2时右侧MCA及ACA均未见血流信号。从T0到T1,造模成功大鼠右脑PI、AUC及WIS降低,TTP、RT延长,差异有统计学意义(P<0.05),但双侧颅内动脉的PSV、EDV和MV的差异无统计学意义。从T1到T2,CEUS定量参数(除外AUC和TPH)没有明显变化,而左侧大脑中动脉(LMCA)及双侧PCA的PSV、EDV及MV差异均有统计学意义(P<0.05),T2时流速高于T1时流速。结论 CEUS和DUS能够较好地反映MCAO大鼠颅内血流动力学与脑组织灌注的变化趋势,提供了一个多时点评估缺血性脑卒中模型大鼠的新手段。Abstract:Objective To assess the value of contrast-enhanced ultrasound (CEUS) and color Doppler ultrasound (DUS) on hemodynamic changes and cerebral perfusion quantitative analyses in Sprague-Dawley (SD) rats with focal permanent ischemic stroke.Methods Sixteen SD rats with thin skulls were subjected to establish middle cerebral artery occlusion (MCAO) model. CEUS images were performed before modeling (T0), immediately after modeling (5-15 min after modeling, T1), 3 h after modeling (T2), followed by the measurement of bilateral middle cerebral artery (MCA), anterior cerebral artery (ACA), posterior cerebral artery (PCA) using DUS. The peak systolic velocity (PSV), end-diastolic velocity (EDV) and mean velocity (MV) of these arteries were obtained. The brain time-intensity curve was taken as interest region of the whole right brain, and the quantitative parameters of CEUS were obtained, including peak intensity (PI), area under the curve (AUC), wash in slope (WIS), time to peak (TTP), rise time (RT) and time from peak to one half (TPH). The modified neurological deficit score (mNSS) of the rats was performed 3 h after the modeling, and the data of the rats with a score of 9-11 were statistically analyzed.Results A total of 12 rats were successfully modeled and completed with mNSS score 9-11. No blood flow signals were observed on the right MCA and ACA in the 12 rats at T1 and T2. From T0 to T1, PI, AUC and WIS of the right hemisphere decreased sharply with TTP and RT significantly prolonged, and the differences were statistically significant. However, there was no significant difference in hemodynamic parameters at that period of time. From T1 to T2, there were no significant changes in CEUS quantitative parameters (except AUC and TPH), while PSV, EDV, MV of LMCA and bilateral PCA showed significant acceleration, and the differences were statistically significant.Conclusion CEUS and DUS can reveal the intracranial hemodynamics and brain tissue perfusion trends of MCAO rats, which could be new methods in assessment of ischemic stroke model at multiple time points.
-
脑卒中是因大脑血液循环障碍导致的脑功能缺损综合征,具有高发病率、高致死率、高致残率和高复发率特点[1],是严重影响国计民生的重要公共卫生问题。急性脑卒中以缺血性脑卒中最多,约占80%。缺血性急性脑卒中的治疗存在明显“时间窗”,对于脑卒中的早期诊断与评估极为重要[2]。线栓法制备的大鼠大脑中动脉闭塞模型(middle cerebral artery occlusion,MCAO)是应用极为广泛、经典的缺血性急性脑卒中动物模型之一[3-4],可用于探讨脑卒中发生发展的机制、新型药物的有效性和安全性等。
超声检查因具有仪器便携、易于获得、价格便宜及无辐射、无创伤等优点,是目前应用最为广泛的影像学检查手段之一。彩色及频谱多普勒模式可以提供血流动力学方面的信息,是评价大血管的有效工具,超声造影(contrast-enhanced ultrasound,CEUS)检查则被认为是评价肝脏、肾脏、心肌、肌肉骨骼系统、睾丸等多种组织器官血流灌注的最有价值的方法之一[5-12],在人体颅脑检查中的价值也得到了验证[12-13]。在动物实验中,由于大鼠颅骨的遮挡,骨缝严密,没有足够的骨窗容超声波穿透,因此鲜有将超声技术应用于大鼠脑卒中模型研究。本研究将大鼠局部颅骨磨薄[14-15]后,通过CEUS及彩色频谱多普勒超声(color Doppler ultrasound,DUS)观察造模前后3个不同时间点的脑血流动力学与脑灌注成像的动态变化过程,探讨超声检查在检测大鼠缺血性脑卒中模型早期发生发展的病理生理机制中的应用价值。
1. 对象与方法
1.1 实验动物
SPF级成年雄性SD大鼠16只(达硕动物实验公司),体质量260~295 g。大鼠置于22 ℃的恒温间分笼饲养,12 h黑暗/明亮循环,自由饮水与进食。术前一晚大鼠禁食不禁饮。该实验方案获得四川大学华西医院动物伦理委员会批准(批准号: 2016037A)。
1.2 磨薄颅骨及MCAO模型的建立
用10%水合氯醛(3.5 mL/kg)腹腔注射麻醉及氟美林(0.03 mL/只)肌肉注射镇痛,参照文献[14-15]方法将大鼠局部颅骨(右侧颅骨约6 mm×6 mm范围)磨薄,随后采用改良线栓法[3, 16]建立大鼠右侧MCAO模型。线栓采用商品化A5级大鼠专用线栓〔直径约0.26 mm的尼龙线栓,头端5 mm包被直径(0.34±0.02) mm硅橡胶,在距顶端18 mm处标记〕。造模术后每只大鼠给予3~5 mL 5%糖盐水腹腔注射,每只大鼠单独置于保温笼,并密切观察至苏醒。造模后3 h行大鼠改良神经功能缺损评分(modified neurological deficit score,mNSS)。
1.3 CEUS及DUS
使用Philips iU 22彩色多普勒超声诊断仪行经颅超声成像,探头为L12-5及L15-7io线阵探头。CEUS使用该超声诊断仪自带的脉冲反转技术,分别于造模前(T0)、造模后即刻(T1,造模后5~15 min)及造模后3 h(T2)时在异氟烷气体麻醉(诱导麻醉浓度4%,持续麻醉浓度2%)下,各进行一次CEUS及DUS成像。
大鼠俯卧于固定台上,颈部垫一厚约1 cm泡沫以保持头颈部呈水平位,无左右偏斜,两前肢自然放置于头部两侧,两后肢自然向后伸展。将L12-5探头垂直于大鼠大脑固定于脑立体定位仪的立臂上,将探头中心移动至大鼠两眼后眦连线约5.5 mm处(视交叉平面),保证探头与大鼠头部良好接触(图1)。保证超声仪主要条件设置固定不变,包括时间补偿增益:均置于最大处;机械指数:0.10;焦点:0.5~0.75 cm;超声造影增益:75%。造影剂(SonoVue®,Bracco,意大利)经尾静脉快速团注(0.15 mL/次),随即快速推注0.3 mL的0.9%生理盐水。存储动态图像30 s。
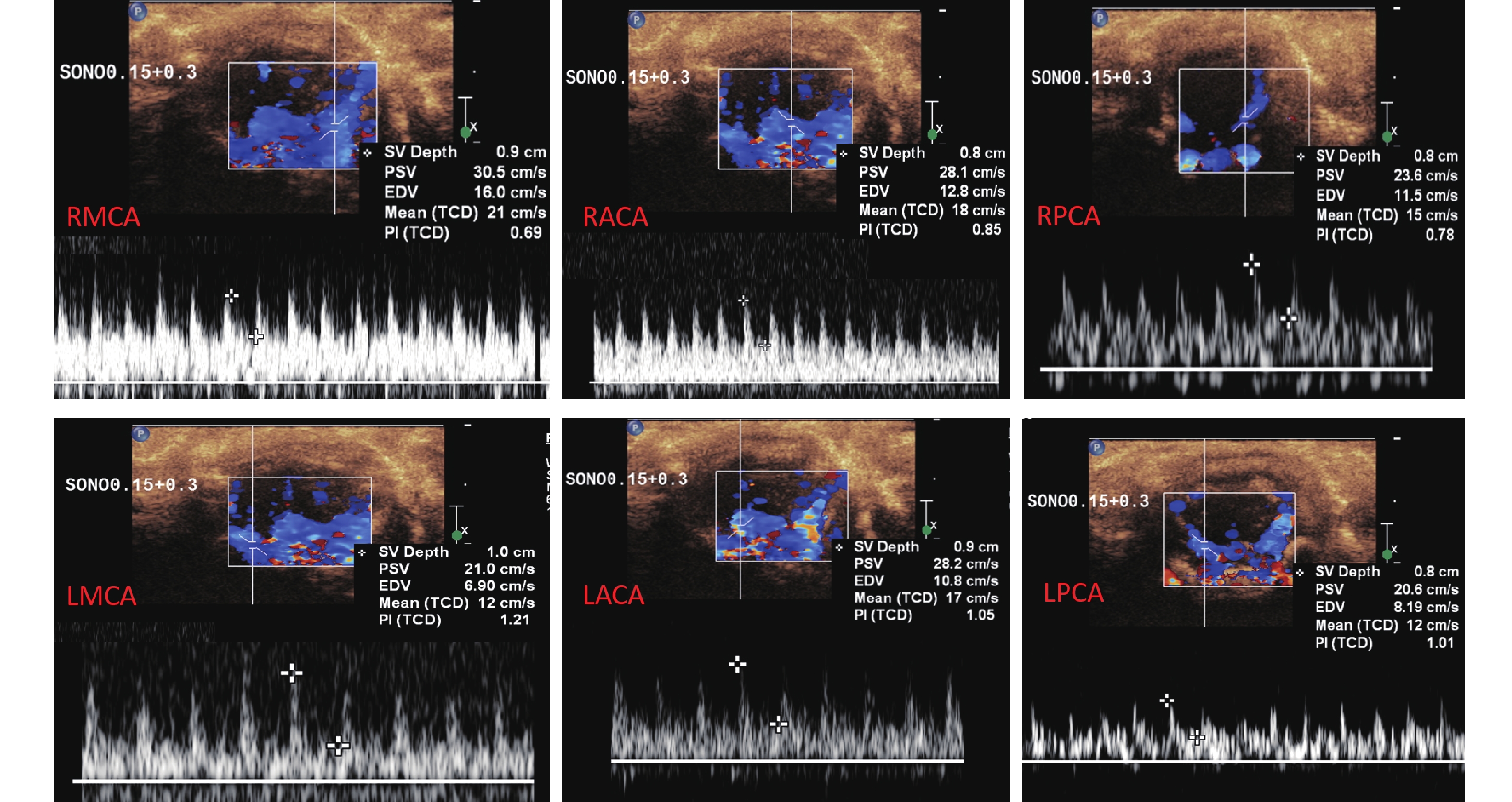
随后迅速切换成L15-7io曲棍球型高频探头,以冠状切面依次迅速探查右侧大脑中动脉(right middle cerebral artery,RMCA)、右侧大脑前动脉(right anterior cerebral artery,RACA)、左侧大脑前动脉(left anterior cerebral artery,LACA)、左侧大脑中动脉(left middle cerebral artery,LMCA)、左侧大脑后动脉(left posterior cerebral artery,LPCA)及右侧大脑后动脉(right posterior cerebral artery,RPCA),测量上述动脉血流频谱,获得各颅内动脉的最大峰值流速(peak systolic velocity,PSV)、舒张末期流速(end-diastolic velocity,EDV)及时间平均的峰值流速(简称平均流速,mean velocity, MV),见图2。测量时保持相同动脉位于相同深度(大脑前动脉、大脑中动脉在0.8~0.9 cm处测量,大脑后动脉在1.0 cm处测量),所有动脉均保持取样角度为50°。保证所有大鼠均采用同样仪器条件设置进行检查(彩色增益:86%,脉冲重复频率:1 625 Hz,壁滤波:65 Hz)。
![]() 图 2 多普勒超声测量大鼠颅内动脉(造模前)Figure 2. Enhanced color doppler ultrasonography for measurement of intracranial artery in rats (before modeling)RMCA:Right middle cerebral artery;RACA:Right anterior cerebral artery;RPCA:Right posterior cerebral artery;LMCA:Left middle cerebral artery;LACA:Left anterior cerebral artery;LPCA:Left posterior cerebral artery
图 2 多普勒超声测量大鼠颅内动脉(造模前)Figure 2. Enhanced color doppler ultrasonography for measurement of intracranial artery in rats (before modeling)RMCA:Right middle cerebral artery;RACA:Right anterior cerebral artery;RPCA:Right posterior cerebral artery;LMCA:Left middle cerebral artery;LACA:Left anterior cerebral artery;LPCA:Left posterior cerebral artery1.4 时间强度曲线(TIC)的定量分析
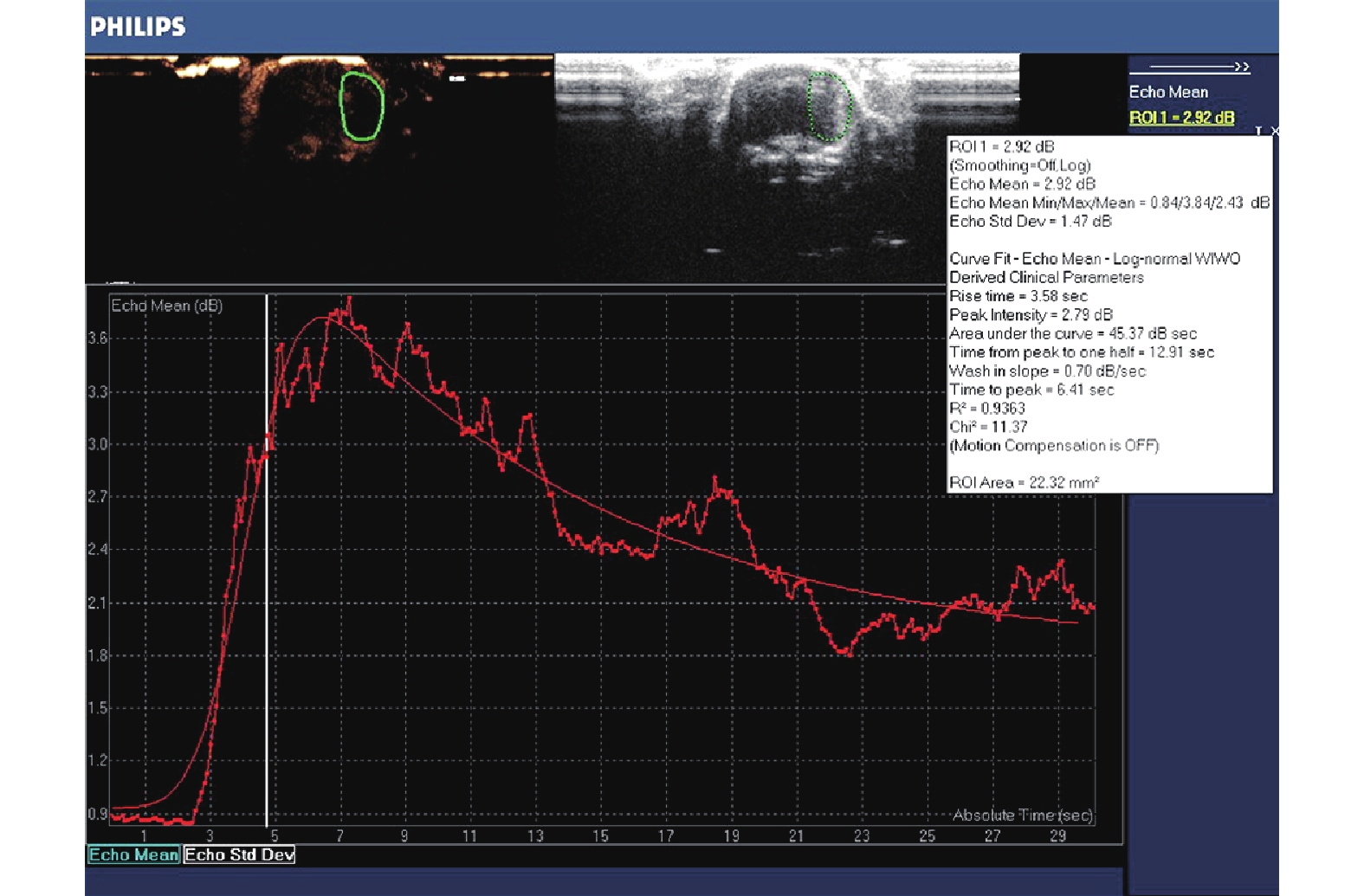
用DICOM格式(包括数字成像和通信两方面内容)存储图像,并使用仪器自带的脱机分析软件QLAB 8.1进行TIC定量分析。打开动态图像,使用自由曲线在右脑内勾勒出感兴趣区(ROI),面积范围约22.0~23.0 mm2,其形态与右侧脑切面类似,外侧缘贴近大鼠的脑实质外侧缘,得到右脑整体脑实质的TIC曲线。使用log-normal WIWO模式拟合曲线,得到6个参数:峰值强度(peak intensity,PI);上升时间(rise time,RT);达峰时间(time to peak,TTP);峰值下降至一半强度的时间(time from peak to one half,TPH);增强斜率(wash in slope,WIS);曲线下面积(area under the curve,AUC),见图3。
1.5 大鼠神经功能缺损评分
造模后3 h,对大鼠进行mNSS[17],即通过对大鼠感觉、运动、反射及平衡能力的综合评分,对其神经功能缺损严重程度进行量化。mNSS总分18分,其中轻度损伤为0~6分,中度损伤为7~12分,重度损伤为13~18分,神经功能损伤程度随分值増大而加重,以神经功能缺损评分为9~11分确认为造模成功。
1.6 统计学方法
所有定量数据使用中位数(四分位间距)表示。采用Friedman检验分别比较T0、T1、T2三个时间点之间的参数,使用符号秩检验(Wilcoxon signed rank test)进行时点间参数的两两比较。α双侧=0.05。
2. 结果
2.1 MCAO造模及实验完成情况
造模后3 h ,mNSS为9~11分确认为造模成功,16只大鼠中12只造模成功,并完成实验。3只大鼠在实验过程中死亡,1只造模失败(发现脑底部血肿,证实为脑出血)。
2.2 T0、T1、T2各时间点血流动力学参数的比较
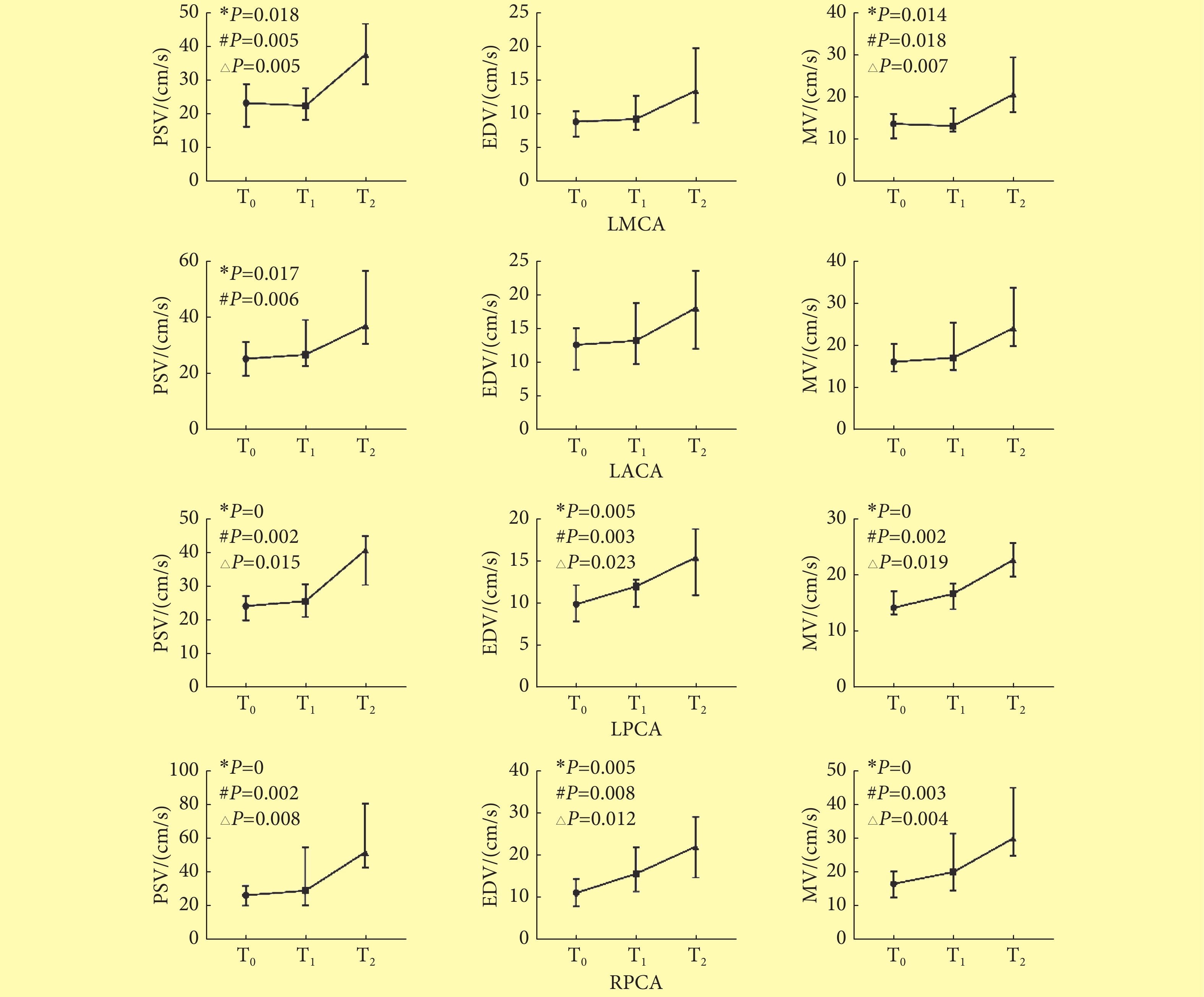
造模成功大鼠在T1及T2时DUS均显示RMCA及RACA血流信号消失。LMCA的PSV与MV、LACA的PSV、双侧PCA的PSV、EDV、MV在3个时点间的差异均有统计学意义。两两比较发现,T0与T1之间各颅内动脉的血流参数差异均无统计学意义。T0与T2比较,除了LMCA的EDV、和LACA的EDV、MV,其余各动脉的PSV、EDV、MV差异均有统计学意义(P<0.05),T2时流速高于T0时流速;T1与T2比较,除LACA各参数差异没有统计学意义之外,LMCA的PSV、MV及双侧PCA的PSV、EDV及MV差异均有统计学意义(P<0.05),T2时流速高于T1时流速。见图4。
![]() 图 4 T0、T1、T2时间点MCAO大鼠颅底动脉血流动力学的动态变化〔中位数(P25~P75), n=12〕Figure 4. Hemodynamics changes of four cerebral arteries in three time points in MCAO rats (median (P25-P75), n=12)T0: Before modeling; T1: 5-15 min after modeling; T2: 3 h after modeling; LMCA: Left middle cerebral artery; LACA: Left anterior cerebral artery; RPCA: Right posterior cerebral artery; LPCA: Left posterior cerebral artery; PSV: Peak systolic velocity; EDV: End-diastolic velocity; MV: Mean velocity; *Difference of this parameter between three time points achieved statistical significance; # Difference of this parameter between T0 and T2 achieved statistical significance; △Difference of this parameter between T1and T2 achieved statistical significance
图 4 T0、T1、T2时间点MCAO大鼠颅底动脉血流动力学的动态变化〔中位数(P25~P75), n=12〕Figure 4. Hemodynamics changes of four cerebral arteries in three time points in MCAO rats (median (P25-P75), n=12)T0: Before modeling; T1: 5-15 min after modeling; T2: 3 h after modeling; LMCA: Left middle cerebral artery; LACA: Left anterior cerebral artery; RPCA: Right posterior cerebral artery; LPCA: Left posterior cerebral artery; PSV: Peak systolic velocity; EDV: End-diastolic velocity; MV: Mean velocity; *Difference of this parameter between three time points achieved statistical significance; # Difference of this parameter between T0 and T2 achieved statistical significance; △Difference of this parameter between T1and T2 achieved statistical significance2.3 T0、T1、T2三个时间点CEUS的TIC曲线参数比较
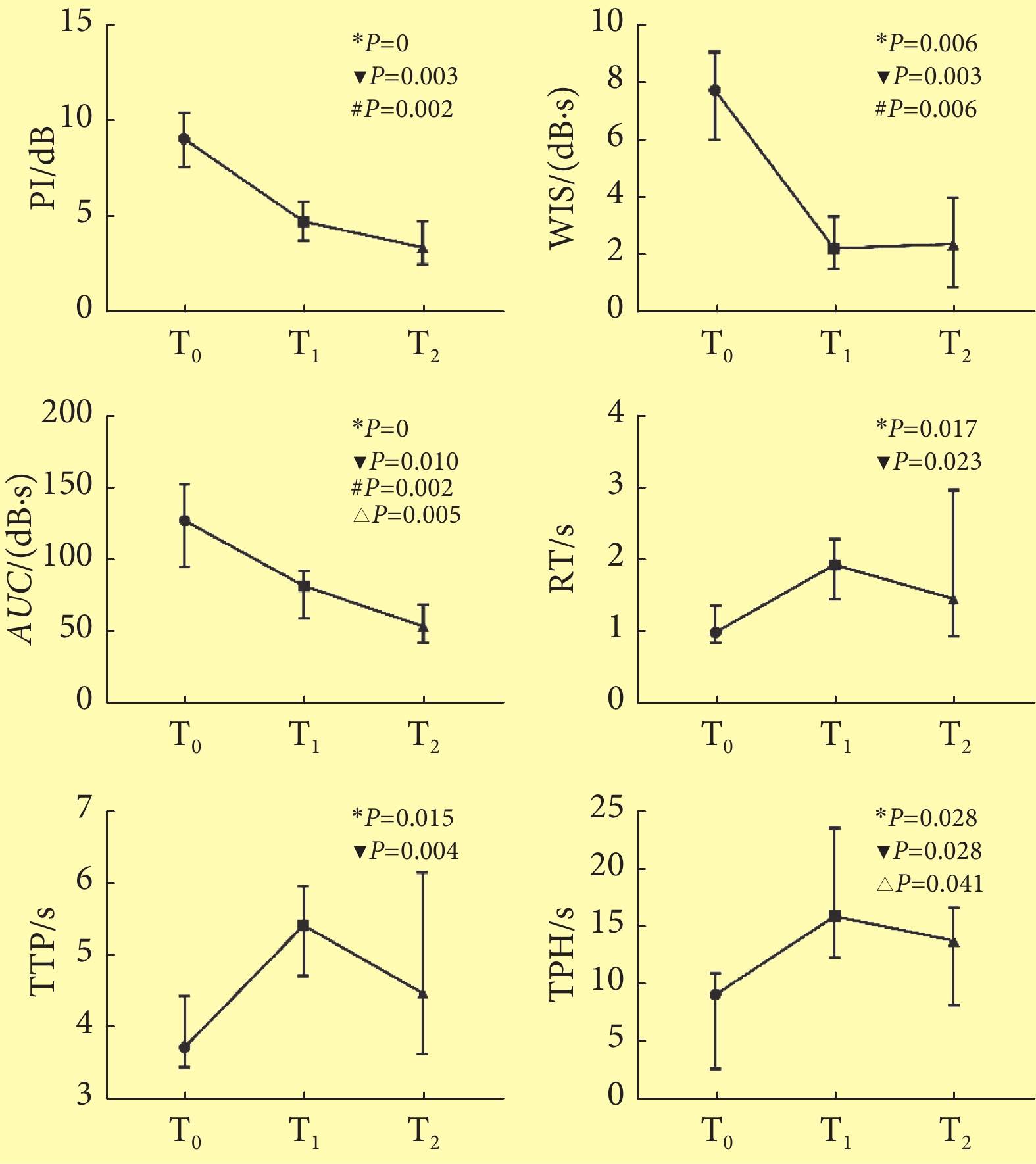
见图5。PI、WIS、AUC、TPH、RT及TTP在3个时间点间的差异均有统计学意义(P<0.05)。两两比较,上述各参数在T0与T1间的变化最为明显,差异均有统计学意义(P<0.05),而从T1至T2间,PI及AUC略微扩大了差异,而RT、WIS、TPH及TTP则是缩小了差异,仅AUC和TPH的差异有统计学意义(P<0.05)。T0与T2间PI、WIS及AUC差异有统计学意义(P<0.05)。
![]() 图 5 三个时间点超声造影时间-强度曲线的各定量参数的比较〔中位数(P25~P75), n=12〕Figure 5. Comparison of quantitative parameters of CEUS in three time points (median (P25-P75), n=12)PI: Peak intensity; AUC: Area under the curve; TTP: Time to peak; WIS: Wash in slope; RT: Rise time; TPH: Time from peak to one half; T0: Before modeling; T1: 5-15 min after modeling; T2: 3 h after modeling; LMCA: Left middle cerebral artery; ▼Difference of this parameter between T0 and T1 achieved statistical significance; #Difference of this parameter between T0 and T2 achieved statistical significance; △Difference of this parameter between T1 and T2 achieved statistical significance; *Difference of this parameter between three time points achieved statistical significance
图 5 三个时间点超声造影时间-强度曲线的各定量参数的比较〔中位数(P25~P75), n=12〕Figure 5. Comparison of quantitative parameters of CEUS in three time points (median (P25-P75), n=12)PI: Peak intensity; AUC: Area under the curve; TTP: Time to peak; WIS: Wash in slope; RT: Rise time; TPH: Time from peak to one half; T0: Before modeling; T1: 5-15 min after modeling; T2: 3 h after modeling; LMCA: Left middle cerebral artery; ▼Difference of this parameter between T0 and T1 achieved statistical significance; #Difference of this parameter between T0 and T2 achieved statistical significance; △Difference of this parameter between T1 and T2 achieved statistical significance; *Difference of this parameter between three time points achieved statistical significance3. 讨论
LAPERGUE等[18]研究认为,经颅DUS是一种有效评估大鼠颅内血流动力学改变的影像学手段,判断MCAO造模是否成功比激光多普勒更有效。而在缺血性脑卒中患者的CEUS脑灌注成像研究发现,联合使用PI与TTP两个指标可以有效检测到梗死灶,敏感性达75%~86%,特异性达96%~100%[19-20]。但在现有发表的文献中鲜有将超声技术应用于大鼠脑卒中模型。
成功的大鼠线栓法MCAO模型需要通过线栓成功堵塞大脑中动脉,但同时也堵塞了同侧大脑前动脉及颈内动脉终末段[3,16]。本研究发现,大鼠线栓法MCAO造模后即刻出现了显著的颅内大动脉血流动力学与脑灌注状态的变化。并且从T0、T1到T2,血流动力学与颅内灌注状态呈现一种规律性的变化。造模成功后即刻,首先迅速出现RMCA与RACA的血流消失,以及右脑内出现低灌注区,PI、AUC及WIS骤然降低,TTP、RT明显升高。而此时LMCA、LACA及双侧PCA的血流动力学变化并不明显。此现象与文献[18]类似,显示了造模成功后堵塞血管的血流消失,及该血管相应供血区即刻的血流量下降,并反映出此时侧支作用尚微弱。造模后3 h,低灌注区持续存在,从T1到T2各定量参数(除AUC)均没有明显变化,而LMCA、LACA及双侧PCA则出现了明显的流速加快。这意味着随着缺血时间的延长,即使没有发生血管再通,大鼠缺血区的血容量也有所改善,考虑应该与侧支循环逐渐开放并开始起代偿作用相关,与CT和磁共振观察到的规律[21]一致。尤其是双侧PCA的流速变化较MCA、ACA更明显,证明了在线栓法MCAO模型中,后循环是比对侧大脑供血动脉更重要的代偿途径,可能是对模型大鼠的预后起最重要作用的定量参数,有进一步研究的价值。既往文献中鲜有将CEUS与DUS结合起来共同用于大鼠脑缺血模型评估者,本研究证明CEUS和DUS能够从颅内血流动力学与脑组织灌注两个方面反映MCAO大鼠的颅内变化趋势,可能对于我们检测及理解模型大鼠的颅内病理生理变化有较高的价值。
由于造模难度较高,本研究样本量较小,尚需要更大样本量的积累与经验总结。在CEUS后进行频谱多普勒的采集,由于使用了造影剂,一方面虽然可以明显提高颅内动脉的显示率,提高血流采集的效率,但另一方面也会出现较明显的血流外溢现象,其测量的血流速度可能出现一定程度的系统误差。因此本实验尽量保证每次检查均在相同条件下、相同时刻进行,以显示血流动力学的变化规律为主要目标。由于后续死亡率较高,我们的观察只进行到造模后3 h。随着技术的不断磨练和改进,我们将继续开展造模后6 h、24 h以及更长时间的观察,更完整的观察记录SD模型大鼠的颅内血流动力学与脑组织灌注的变化规律,将有助于对脑卒中病理生理机制的理解及使用新型药物治疗后的评估。
综上所述,多个时间点的DUS及CEUS成像能够提供SD大鼠局灶性缺血性脑卒中模型的血流动力学和脑灌注状态改变的丰富信息。本研究发现,T1到T2的多普勒血流参数出现了明显差异,T2时未发生梗塞的动脉流速明显加快,可能反映了大动脉随时间逐渐增加的代偿状态;而T0到T1的CEUS参数差异最为明显,反映了颅内灌注的明显降低,而T1到T2时部分参数有所改善,可能提示着侧支代偿的存在。因此该超声成像技术提供了一个多时点评估缺血性脑卒中模型大鼠的新手段,其价值值得进一步探索和挖掘。
-
图 2 多普勒超声测量大鼠颅内动脉(造模前)
Figure 2. Enhanced color doppler ultrasonography for measurement of intracranial artery in rats (before modeling)
RMCA:Right middle cerebral artery;RACA:Right anterior cerebral artery;RPCA:Right posterior cerebral artery;LMCA:Left middle cerebral artery;LACA:Left anterior cerebral artery;LPCA:Left posterior cerebral artery
图 4 T0、T1、T2时间点MCAO大鼠颅底动脉血流动力学的动态变化〔中位数(P25~P75), n=12〕
Figure 4. Hemodynamics changes of four cerebral arteries in three time points in MCAO rats (median (P25-P75), n=12)
T0: Before modeling; T1: 5-15 min after modeling; T2: 3 h after modeling; LMCA: Left middle cerebral artery; LACA: Left anterior cerebral artery; RPCA: Right posterior cerebral artery; LPCA: Left posterior cerebral artery; PSV: Peak systolic velocity; EDV: End-diastolic velocity; MV: Mean velocity; *Difference of this parameter between three time points achieved statistical significance; # Difference of this parameter between T0 and T2 achieved statistical significance; △Difference of this parameter between T1and T2 achieved statistical significance
图 5 三个时间点超声造影时间-强度曲线的各定量参数的比较〔中位数(P25~P75), n=12〕
Figure 5. Comparison of quantitative parameters of CEUS in three time points (median (P25-P75), n=12)
PI: Peak intensity; AUC: Area under the curve; TTP: Time to peak; WIS: Wash in slope; RT: Rise time; TPH: Time from peak to one half; T0: Before modeling; T1: 5-15 min after modeling; T2: 3 h after modeling; LMCA: Left middle cerebral artery; ▼Difference of this parameter between T0 and T1 achieved statistical significance; #Difference of this parameter between T0 and T2 achieved statistical significance; △Difference of this parameter between T1 and T2 achieved statistical significance; *Difference of this parameter between three time points achieved statistical significance
-
[1] RAFII M S, HILLIS A E. Compendium of cerebrovascular diseases. Int Rev Psychiatry,2006,18(5): 395–407. DOI: 10.1080/09540260600935405
[2] JIN H, ZHU S, WEI J W, et al. Factors associated with prehospital delays in the presentation of acute stroke in urban China. Stroke,2012,43(2): 362–370. DOI: 10.1161/STROKEAHA.111.623512
[3] HERSON P S, TRAYSTMAN R J. Animal models of stroke: translational potential at present and in 2050. Future Neurol,2014,9(5): 541–551. DOI: 10.2217/fnl.14.44
[4] ZHANG L, ZHANG R L, JIANG Q, et al. Focal embolic cerebral ischemia in the rat. Nat Protoc,2015,10(4): 539–547. DOI: 10.1038/nprot.2015.036
[5] RIDOLFI F, ABBATTISTA T, BUSILACCHI P, et al. Contrast-enhanced ultrasound evaluation of hepatic microvascular changes in liver diseases. World J Gastroenterol,2012,18(37): 5225–5230. DOI: doi:10.3748/wjg.v18.i37.5225
[6] QUAIA E, NOCENTINI A, TORELLI L. Assessment of a new mathematical model for the computation of numerical parameters related to renal cortical blood flow and fractional blood volume by contrast-enhanced ultrasound. Ultrasound Med Biol,2009,35(4): 616–627. DOI: 10.1016/j.ultrasmedbio.2008.10.003
[7] PORTER T R, XIE F. Myocardial perfusion imaging with contrast ultrasound. JACC Cardiovasc Imaging,2010,3(2): 176–187. DOI: 10.1016/j.jcmg.2009.09.024
[8] WEBER M A, KRIX M, JAPPE U, et al. Pathologic skeletal muscle perfusion in patients with myositis: detection with quantitative contrast-enhanced US—initial results. Radiology,2006,238(2): 640–649. DOI: 10.1148/radiol.2382041822
[9] CHRISTIANSEN J P, LEONG-POI H, AMISS L R, et al. Skin perfusion assessed by contrast ultrasound predicts tissue survival in a free flap model. Ultrasound Med Biol,2002,28(3): 315–320. DOI: 10.1016/S0301-5629(01)00523-3
[10] KLAUSER A, DEMHARTER J, DE MARCHI A, et al. Contrast enhanced gray-scale sonography in assessment of joint vascularity in rheumatoid arthritis: results from the IACUS study group. Eur Radiol,2005,15(12): 2404–2410. DOI: 10.1007/s00330-005-2884-9
[11] CHEN L, ZHAN W W, SHEN Z J, et al. Blood perfusion of the contralateral testis evaluated with contrast-enhanced ultrasound in rabbits with unilateral testicular torsion. Asian J Androl,2009,11(2): 253–260. DOI: 10.1038/aja.2008.13
[12] KASPAR M, PARTOVI S, ASCHWANDEN M, et al. Assessment of microcirculation by contrast-enhanced ultrasound: a new approach in vascular medicine. Swiss Med Wkly, 2015,145:w14047[2019-09-08]. https://doi.org/10.4414/smw.2015.14047.
[13] HWANG M, SRIDHARAN A, DARGE K, et al. Novel quantitative contrast-enhanced ultrasound detection of hypoxic ischemic injury in neonates and infants: pilot study 1. J Ultrasound Med,2019,38(8): 2025–2038. DOI: 10.1002/jum.14892
[14] ERRICO C, PIERRE J, PEZET S, et al. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature,2015,527(7579): 499–502. DOI: 10.1038/nature16066
[15] URBAN A, MACE E, BRUNNER C, et al. Chronic assessment of cerebral hemodynamics during rat forepaw electrical stimulation using functional ultrasound imaging. Neuroimage,2014,101: 138–149. DOI: 10.1016/j.neuroimage.2014.06.063
[16] LOURBOPOULOS A, KARACOSTAS D, ARTEMIS N, et al. Effectiveness of a new modified intraluminal suture for temporary middle cerebral artery occlusion in rats of various weight. J Neurosci Methods,2008,173(2): 225–234. DOI: 10.1016/j.jneumeth.2008.06.018
[17] WANG C, LI F, GUAN Y, et al. Bone marrow stromal cells combined with oxiracetam influences the expression of B-cell lymphoma 2 in rats with ischemic stroke. J Stroke Cerebrovasc Dis,2014,23(10): 2591–2597. DOI: 10.1016/j.jstrokecerebrovasdis.2014.05.035
[18] LAPERGUE B, DEROIDE N, POCARD M, et al. Transcranial duplex sonography for monitoring circle of Willis artery occlusion in a rat embolic stroke model. J Neurosci Methods,2011,197(2): 289–296. DOI: 10.1016/j.jneumeth.2011.02.016
[19] FEDERLEIN J, POSTERT T, MEVES S, et al. Ultrasonic evaluation of pathological brain perfusion in acute stroke using second harmonic imaging. J Neurol Neurosurg Psychiatry,2000,69(5): 616–622. DOI: 10.1136/jnnp.69.5.616
[20] SEIDEL G, ALBERS T, MEYER K, et al. Perfusion harmonic imaging in acute middle cerebral artery infarction. Ultrasound Med Biol,2003,29(9): 1245–1251. DOI: 10.1016/S0301-5629(03)01016-0
[21] KIM J H, SHIN T, CHUNG J D, et al. Temporal pattern of blood volume change in cerebral infarction: evaluation with dynamic contrast-enhanced T2*-weighted MR imaging. AJR Am J Roentgenol,1998,170(3): 765–770. DOI: 10.2214/ajr.170.3.9490971
-
期刊类型引用(2)
1. 李祥龙,陈香州,雷围程,李立东,李尧,杨福兵,杨晓波. 建立与青年大鼠神经功能损伤程度相同的老年大鼠大脑中动脉阻塞模型. 西南医科大学学报. 2023(03): 213-216 .  百度学术
百度学术
2. 娄惠娟,丛德毓,张红石,蒙蒙,李湛新,王宇峰. 局灶性脑缺血动物模型建立及评价概况. 吉林中医药. 2023(10): 1117-1122 .  百度学术
百度学术
其他类型引用(0)

 首页
首页


 下载:
下载: