Constructing Brain Aβ-Targeting Nanoparticles Loaded with EGCG for Treating Alzheimer’s Disease in Mice
-
摘要:目的 构建一种能够进入脑组织且靶向β淀粉样蛋白(β-amyloid, Aβ)的表面修饰多肽序列PTLHTHNRRRRR(简称RD2肽)的纳米递释系统。选择荷载表没食子儿茶素没食子酸酯(epigallocatechin-3-gallate, EGCG),以验证该靶向递释系统对阿尔茨海默病(Alzheimer’s disease, AD)治疗的可行性。方法 制备载EGCG的纳米粒(NP/EGCG)、表面修饰RD2肽的NP/EGCG(RD2-NP/EGCG)和表面修饰RD2肽的未载药纳米粒(RD2-NP),并对其进行表征。硫磺素T实验考察RD2-NP与Aβ的结合能力,离体成像观察RD2-NP在脑病变部位的分布。将寡聚态Aβ42注入ICR小鼠双侧海马内构建AD小鼠模型,AD小鼠分别尾静脉注射生理盐水、EGCG溶液、NP/EGCG或RD2-NP/EGCG共28 d,水迷宫实验评价各组小鼠的空间记忆能力;RT-PCR法测定小鼠海马中肿瘤坏死因子-α(tumor necrosis factor-α, TNF-α)和白介素-1β(interleukin-1β, IL-1β)的mRNA水平;尼氏染色观察海马神经元的形态变化;HE染色观察心、肝、脾、肺、肾的病理学改变。结果 制得的RD2-NP/EGCG粒径为(204.83±2.80) nm,Zeta电位约为−23.88 mV,包封率为94.39%,载药量为5.90%,RD2肽修饰对纳米粒的理化特性无显著影响。RD2-NP具有良好的Aβ结合能力,能浓集于海马和大脑皮质。RD2-NP/EGCG治疗4周能够显著降低AD模型小鼠脑中炎症因子TNF-α和IL-1β水平,修复神经元损伤,改善空间记忆障碍,且无器官毒性。结论 表面修饰RD2肽纳米递释系统可高效递送药物到AD病变部位,改善EGCG对AD的治疗效果。Abstract:Objective To construct a nanodelivery system surface-modified with RD2 peptide (polypeptide sequence PTLHTHNRRRRR) for brain tissue penetration and β-amyloid (Aβ) binding. Epigallocatechin-3-gallate (EGCG) was selected for encapsulation in the targeted delivery system and its therapeutic potential for Alzheimer’s disease (AD) was investigated.Methods EGCG-load nanoparticles (NP/EGCG), NP/EGCG with RD2 peptide surface modification (RD2-NP/EGCG), as well as RD2 peptide-modified blank nanoparticles (RD2-NP) were prepared and characterized. Thioflavin T assay was done to assess the ability of RD2-NP to bind with Aβ and ex vivo imaging was conducted to evaluate the distribution of RD2-NP in brain lesion sites. The AD mice model was established by injecting oligomeric Aβ42 in the bilateral hippocampi of ICR mice. Then AD mice were administered intravenously through the tail vein with normal saline, EGCG solution, NP/EGCG or RD2-NP/EGCG for 28 d, respectively, and the Morris water maze tests were performed to assess the spatial memory of mice. Subsequently, RT-PCR method was used to determine the mRNA levels of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in the hippocampus of the mice, and the morphological changes of hippocampal neurons were observed with Nissl staining. Additionally, the pathological changes of heart, liver, spleen, lung, and kidney were characterized by hematoxylin-eosin (HE) staining.Results The particle diameter of the prepared RD2-NP/EGCG was (204.83±2.80) nm and the zeta potential was −23.88 mV. The encapsulation efficiency and drug loading capacity were 94.39% and 5.90%, respectively. The RD2 peptide modification has no significant effect on the physiochemical properties of the nanoparticles. RD2-NP had good Aβ binding ability, and it could be concentrated in hippocampus and cerebral cortex, the most common Aβ deposition sites. The four-week RD2-NP/EGCG treatment significantly decreased the expression of the pro-inflammatory cytokine TNF-α and IL-1β, restored neuronal losses and hippocampal damage, and ameliorated spatial memory impairment in AD model mice. Moreover, treatment with the RD2-NP/EGCG did not present organ toxicity.Conclusion Surface modified RD2 peptide nanodelivery system can efficiently deliver drugs to AD lesions and improve the therapeutic effect of EGCG on AD.
-
阿尔茨海默病(Alzheimer’s disease, AD)是常见的神经退行性疾病,主要表现为进行性认知障碍和记忆减退[1]。目前AD的治疗面临很大困难,这主要有两方面原因:①AD的发病机制异常复杂且神经病变症状出现较晚,AD的早期诊断和治疗难度很大[2];②由于血脑屏障的阻碍以及药物入脑后呈全脑分布,绝大部分药物难以在AD病变部位达到有效治疗浓度,从而极大地限制了药物治疗作用的发挥[3]。
近年来,纳米技术的发展为“精准递送”药物到达病变部位提供了有效手段。纳米载体可以在合适的靶向功能基介导下浓集于病变部位,从而提高药物作用的专一性,降低全身分布引起的不良反应[4- 5]。AD的主要神经病理特征包括淀粉斑和神经纤维缠结[6]。其中,存在于神经细胞外的淀粉斑被普遍认为是最恰当的AD分子生物标记,其主要成分为β淀粉样蛋白42(β-amyloid 42, Aβ42)(约占96%)。多肽序列PTLHTHNRRRRR(简称RD2肽)是一条与Aβ42有高亲和性的d型十二肽,其序列中含多个碱性氨基酸,能透过血脑屏障。研究已证实,RD2肽治疗可以改善APP/PS1转基因小鼠的记忆障碍[7]。绿茶多酚活性成分表没食子儿茶素没食子酸酯(epigallocatechin-3-gallate, EGCG)已被证实具有清除氧自由基,抑制Aβ产生和Tau蛋白磷酸化,抗炎以及神经保护作用。然而,EGCG口服易被外排,入脑量少,不能有效治疗脑部疾病[8]。因此,本研究选择RD2肽作为靶向脑淀粉斑的功能基,同时选择生物相容性良好的聚乙二醇-聚乳酸(PEG-PLA)嵌段聚合物作为纳米材料,构建一种靶向脑淀粉斑的载EGCG的纳米递释系统,考察该系统能否提高模型药物EGCG在AD病变部位的浓集以及对AD的治疗效果。
1. 材料和方法
1.1 主要材料、试剂和仪器
MePEG-PLA(相对分子质量50 000)和Mal-PEG-PLA(相对分子质量70 000)由华东理工大学实验室合成;RD2肽(PTLHTHNRRRRR, MW1798)和Aβ42由中肽生化有限公司合成;EGCG(批号:N1027A;纯度≥95%)购自大连美仑生物技术有限公司;硫磺素T、聚乙烯醇(PVA)、1,1,1,3,3,3,-六氟-2-丙醇(HFIP)购自Sigma-Aldrich公司;1,1′-二十八烷基-3,3,3′,3′-四甲基吲哚碳酰碘化物(1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide, DiR)染料购自Biotium公司;肿瘤坏死因子-α(tumor necrosis factor-α, TNF-α)、白介素-1β(interleukin-1β, IL-1β)、β-actin引物由生工生物工程(上海)有限公司合成;Hifair® Ⅱ1st Strand cDNA Synthesis Kit、qPCR SYBR Green Master Mix购自上海翊圣生物科技有限公司;尼氏染色试剂盒购自北京索莱宝科技有限公司;HE染色试剂盒购自武汉博士德生物工程有限公司。
恒温磁力搅拌仪(武汉赛维尔生物,中国),冷冻离心机5418R(Eppendorf,德国),激光粒度仪Nano-ZS(Malvern,英国);小动物活体成像(IVIS Spectrum, CailperPerkinElemer,美国),多功能酶标仪Synergy H1(Bio-tek,美国),小鼠水迷宫视频追踪系统(WaterMaze, Coulbourn,美国),PCR仪(Applied Biosystems,美国)。
1.2 实验动物
ICR小鼠(雄性,体质量18~20 g)购自上海西普尔-必凯实验动物限公司。3×Tg-AD转基因小鼠,品系:B6;129-Psen1tm1Mpm Tg(AβPPSwe, tauP301L)1Lfa/Mmjax,10月龄,雌性,购于美国Jackson Laboratory。本研究已通过复旦大学动物伦理委员会的伦理审查并备案,实验过程遵循我国《实验动物福利伦理审查指南(GB/T 358922018)》提出的原则与要求。
1.3 方法
1.3.1 载荧光探针DiR的纳米粒制备
采用乳化/溶剂蒸发法制备载DiR的PEG-PLA纳米粒[9]。称取14 mg MePEG-PLA和6 mg Mal-PEG-PLA溶解于1 mL二氯甲烷中,加入20 μg DiR混合均匀。加入质量分数1%胆酸钠水溶液3 mL,200 W、间断超声60 s,将所得初乳转移入20 mL质量分数0.5%胆酸钠水溶液中,搅拌5 min,室温旋转蒸发除去二氯甲烷。纳米悬液过Sepharose CL-4B凝胶柱洗脱除去游离的DiR,离心即得NP/DiR。
将RD2肽溶于去离子水中,按RD2肽与Mal-PEG-PLA摩尔比1∶1的量加入纳米粒溶液中,混合搅拌4 h,即得RD2修饰的纳米粒(RD2-NP/DiR)。
1.3.2 RD2-NP/EGCG的制备
EGCG可溶于水,不适宜用乳化/溶剂蒸发法制备载药纳米粒。因此,参照文献,采用共沉淀法制备载EGCG的纳米粒[10]。称取11.2 mg MePEG-PLA和4.8 mg Mal-PEG-PLA溶于200 μL二甲亚砜(dimethyl sulfoxide, DMSO)中配成材料溶液。另称取1 mg EGCG溶于100 μL DMSO中配成药物溶液。将两者涡旋混合均匀,边搅拌边滴加入质量分数2%PVA溶液中。继续搅拌20 min,200 W超声分散30 s,随后继续搅拌3 h除去有机溶剂。离心、洗除未包封的EGCG,即得NP/EGCG。按1.3.1方法在纳米粒表面连接RD2肽,得到RD2-NP/EGCG。
1.3.3 载EGCG纳米粒的表征
样品用去离子水适当稀释后,采用Malvern激光粒度/Zeta电位测定仪检测纳米粒的粒径与Zeta电位。
取纳米粒溶液,加入2倍体积的乙腈,涡旋混合使聚合物材料溶解、纳米粒破坏,释放包封的EGCG,12 000 r/min、4 ℃离心10 min,取上清液HPLC分析,计算纳米粒的载药量(DLC)和包封率(EE)。DLC(%)=纳米粒中包封的EGCG量/纳米粒总质量×100%;EE(%)=纳米粒中包封的EGCG量/投药量×100%
1.3.4 硫磺素-T(Thioflavin T, Th-T)荧光分析法考察RD2-NP与Aβ的结合能力
Th-T测定法是一种可视化和量化Aβ纤维化的常用方法。Aβ单体在缓冲液中老化聚集形成Aβ纤维,该纤维能与Th-T特异结合产生荧光,且荧光强度与Aβ纤维量呈正相关。文献报道[7],RD2肽与Aβ单体具有亲和力,可阻碍Aβ纤维形成,导致Aβ纤维与Th-T的结合减弱,荧光强度降低。因此荧光强度的变化可以评估修饰了RD2肽的PEG-PLA纳米粒是否具有与Aβ的结合能力。
首先配制Aβ42单体溶液:取Aβ42冻干粉在HFIP中溶解过夜,再次冻干去除HFIP并溶解在pH7.4的10 mmol/L磷酸钠缓冲液中,即得Aβ42单体溶液。
取20 μmol/L Aβ42单体溶液,加入80 μmol/L RD2-NP(未载药纳米粒,参照1.3.2方法制备)和5 μmol/L Th-T,置于荧光微孔板中混匀,在37 ℃条件下共孵育,分别于2、4、8、20和24 h用酶标仪测定Th-T的荧光强度。Th-T的激发波长(excitation wavelength, Ex)为440 nm,发射波长(emission wavelength, Em)为490 nm。
1.3.5 脑离体成像观察
取7只3×Tg-AD转基因小鼠,分别尾静脉注射NP/DiR(n=3)或RD2-NP/DiR(n=3),剂量均为含0.5 mg DiR/kg,空白对照组注射相同体积的生理盐水(n=1)。给药后1 h,腹腔注射5%水合氯醛麻醉小鼠,心脏灌流生理盐水,取全脑,分离大脑皮质和海马,采用小动物活体成像系统进行成像观察并拍照(Ex=748 nm,Em=780 nm)。
1.3.6 AD小鼠模型的构建和分组干预
精密称取2 mg Aβ42,加入30 μL DMSO溶解,再加入1 970 μL生理盐水稀释至1 mg/mL。37 ℃密闭孵育7 d,使其老化聚集形成Aβ42寡聚体。按文献报道方法构建AD小鼠模型[11],即将老化的Aβ42(5 μL)注入ICR小鼠双侧海马内(以前囟坐标为原坐标,旁开1.8 mm,后2.3 mm,深2 mm),假手术组海马内注射生理盐水。手术后小鼠置于远红外理疗灯下以利于其苏醒。小鼠苏醒后常规饲养。
常规饲养7 d后,将小鼠分为5组(其中AD模型小鼠随机分为4组,即②~⑤),每组9只:①假手术对照组(Sham组);② AD对照组(AD组);③ EGCG溶液组;④ NP/EGCG组;⑤ RD2-NP/EGCG组。①、②组每日给予100 μL生理盐水;③、④、⑤组分别给予EGCG、NP/EGCG、RD2-NP/EGCG,溶剂均为生理盐水,给药剂量均按小鼠体质量换算为每日2 mg EGCG/kg。每组每天给药一次,连续尾静脉注射给药28 d,第23天开始进行Morris水迷宫实验(见1.3.7)。给药28 d后,处死小鼠,取脑组织进行脑炎性因子mRNA水平测定(见1.3.8)和病理染色(见1.3.9)。
1.3.7 Morris水迷宫行为学实验
为了考察RD2-NP/EGCG对小鼠认知和记忆力的改善情况,我们进行了水迷宫行为学实验。行为学考察包括定位航行实验和空间探索实验[12]。定位航行实验持续6 d,各组小鼠每天经过4个象限的入水训练,寻找水池中的平台,记录小鼠每天寻台时间(潜伏期)的变化。第6天训练结束后,撤去水下平台,进行空间探索实验,采用图像采集软件捕捉小鼠在60 s内游泳轨迹,统计小鼠跨平台次数和在目标象限的停留时间。
1.3.8 脑炎性因子mRNA水平测定
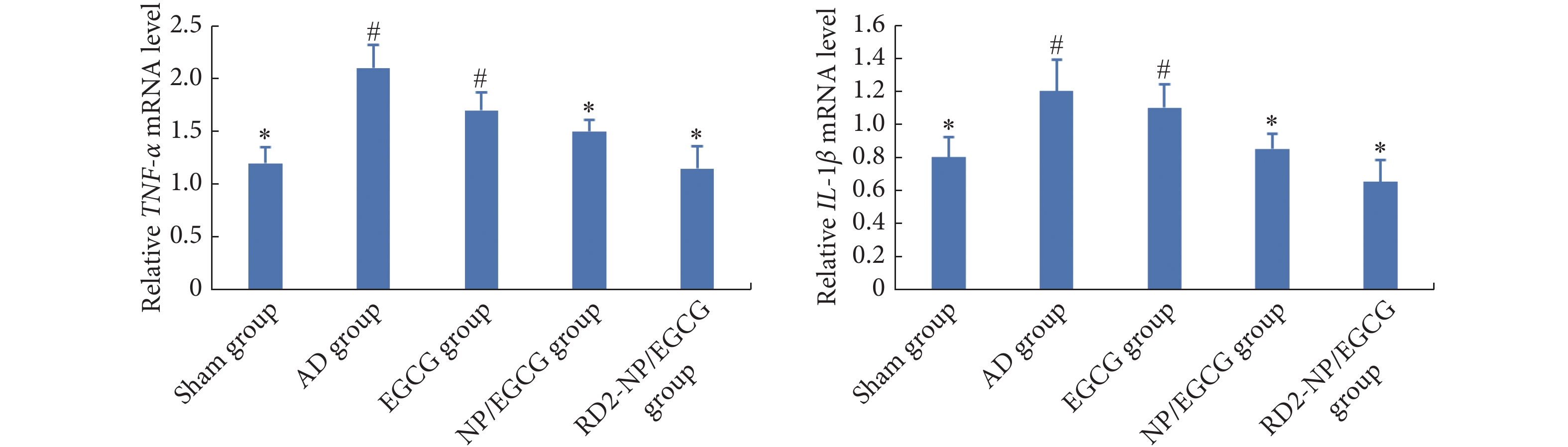
为了验证RD2-NP/EGCG对神经炎症的抑制作用,我们通过RT-PCR测定了两种主要炎性细胞因子IL-1β和TNF-α mRNA水平。行为学实验结束后,麻醉小鼠、心脏灌注生理盐水,立即剥离双侧海马,置于1 mL冰冷的Trizol试剂中匀浆,提取总RNA。然后使用逆转录试剂盒(Hifair® II1st Strand cDNA Synthesis Kit)将提取的总RNA反转录为cDNA。再按试剂盒说明使用SYBR Green PCR检测方法,在iQ5多色实时PCR检测系统上进行RT-PCR实验。引物序列(均为5′–3′)为,TNF-α,F:AGGCGGTGCCTATGTCTCA,R:GAGGCCATTTGGGAACTTCT;IL-1β,F:GAAATGCCACCTTTTGACAGTG,R:CTGGATGCTCTCATCAGGACA;β-actin,F:CCACACCCCGCCAGTTC,R:GACCCATACCCACCATCACACC。以β-actin作为内参,采用2−ΔΔCt法计算TNF-α、IL-1β的mRNA的相对表达水平。
1.3.9 尼氏染色和HE染色
行为学测试结束后,小鼠麻醉,采用生理盐水和体积分数4%多聚甲醛心脏灌注。摘取小鼠的脑、心、肝、脾、肺和肾,放入体积分数4%多聚甲醛中固定48 h,用于石蜡切片,切片厚度为6 μm。随后采用尼氏染色试剂盒对脑切片进行尼氏染色,观察各组小鼠脑切片海马CA1区神经元形态。使用HE染色试剂盒对心、肝、脾、肺、肾切片进行HE染色,观察递药系统的安全性。
1.3.10 统计学方法
除另有说明外,数据均以
$ \bar x \pm s $ 表示。各组之间比较采用单因素方差分析(one-way ANOVA),两组比较采用非配对双尾t检验,P<0.05为差异有统计学意义。2. 结果
2.1 载EGCG纳米粒的表征
制得的NP/EGCG平均粒径为(198.54±3.67) nm,纳米粒表面修饰RD2肽后,粒径略增大,为(204.83±2.80) nm,但差异无统计学意义。RD2-NP/EGCG的Zeta电位、包封率、载药量与NP/EGCG的相应特征差异无统计学意义。见表1。
2.2 硫磺素-T荧光分析
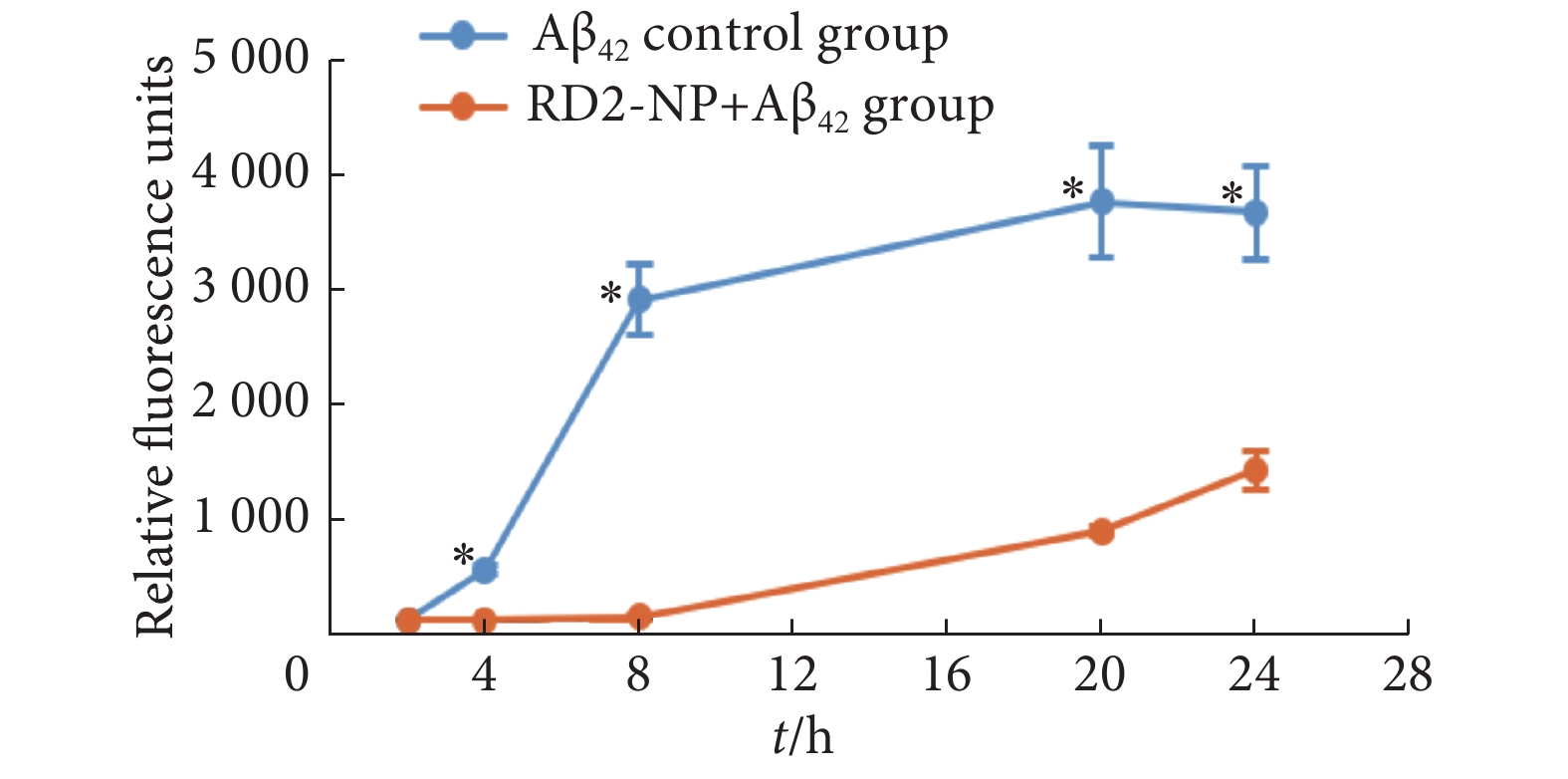
如图1所示,随着孵育时间延长,Aβ对照组的荧光强度不断增加,证明形成了Aβ纤维。而RD2-NP与Aβ42孵育24 h,荧光强度仅为Aβ对照组的37.10%,显示RD2-NP能显著抑制Aβ42的聚集。此实验结果表明RD2肽修饰的PEG-PLA纳米粒仍具有较强的Aβ结合能力,能够抑制Aβ的聚集。
表 1 不同纳米粒的理化性质(n=3)Table 1. Physicochemical characterization of different nanoparticles (n=3)Nanoparticles Size/nm PDI Zeta potential/mV DLC/% EE/% NP/EGCG 198.54±3.67 0.185±0.042 −23.51±0.32 95.43±1.02 5.96±0.06 RD2-NP/EGCG 204.83±2.80 0.191±0.066 −23.88±1.24 94.39±0.86 5.90±0.05 PDI: Polydispersity index; DLC: Drug loading capacity; EE: Encapsulation efficiency. ![]() 图 1 Th-T分析RD2-NP对Aβ42聚集的影响(n=3)Figure 1. The influence of RD2-NP on Aβ42 aggregation was analyzed with Thioflavin T assay (n=3)Binding to Aβ42 was determined using the Thioflavin T fluorescence at 440 nm excitation wavelength and 490 nm emission wavelength and presented in relative fluorescence units. *P<0.05, vs. RD2-NP+Aβ42 group.
图 1 Th-T分析RD2-NP对Aβ42聚集的影响(n=3)Figure 1. The influence of RD2-NP on Aβ42 aggregation was analyzed with Thioflavin T assay (n=3)Binding to Aβ42 was determined using the Thioflavin T fluorescence at 440 nm excitation wavelength and 490 nm emission wavelength and presented in relative fluorescence units. *P<0.05, vs. RD2-NP+Aβ42 group.2.3 离体成像观察RD2-NP/DiR在AD小鼠病变部位的分布
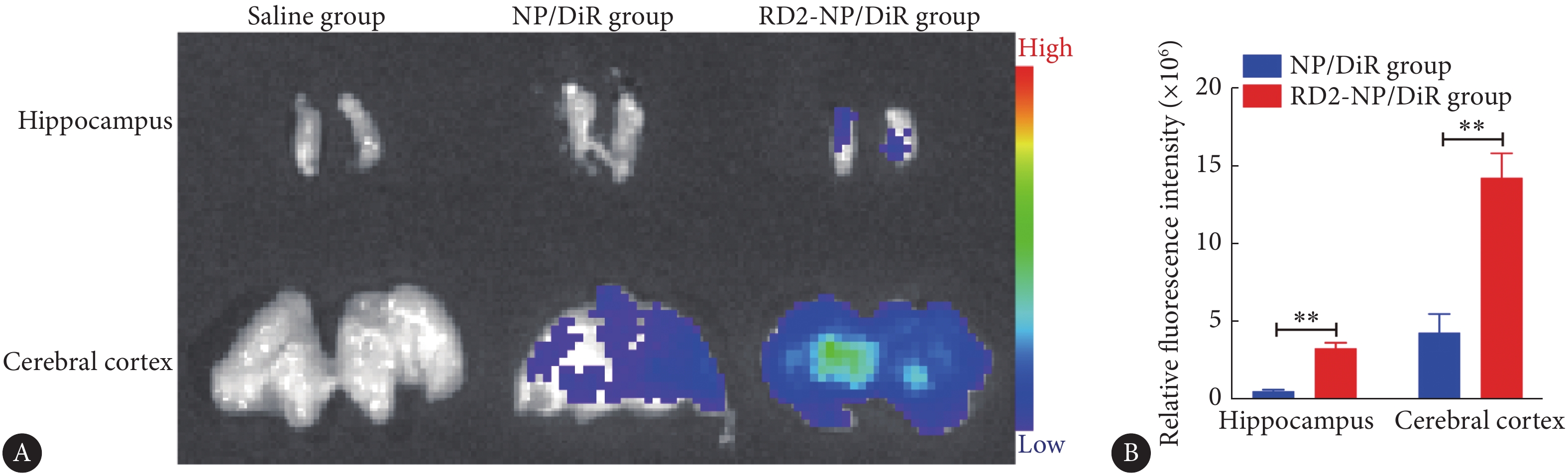
3×Tg-AD小鼠尾静脉注射纳米粒1 h后,脑离体成像结果如图2所示。相较于NP/DiR组,RD2-NP/DiR组在AD主要病变区域大脑皮质和海马的荧光明显增强,分别为NP/DiR组的3.32倍和6.34倍,表明RD2-NP/DiR能浓集于富含淀粉斑的AD病变部位,这将有利于EGCG治疗作用的发挥。
![]() 图 2 离体成像观察纳米粒在脑病变部位(转基因鼠的海马和大脑皮质)的分布和相对荧光强度A: Ex vivo imaging of hippocampus and cerebral cortex; B: Relative fluorescence intensity of hippocampus and cerebral cortex from each mice group (n=3). **P<0.01.Figure 2. The ex vivo imaging of brain lesions of 3×Tg-AD transgenic mice at 1 h after intravenous injection of DiR-loaded NP and RD2-NP
图 2 离体成像观察纳米粒在脑病变部位(转基因鼠的海马和大脑皮质)的分布和相对荧光强度A: Ex vivo imaging of hippocampus and cerebral cortex; B: Relative fluorescence intensity of hippocampus and cerebral cortex from each mice group (n=3). **P<0.01.Figure 2. The ex vivo imaging of brain lesions of 3×Tg-AD transgenic mice at 1 h after intravenous injection of DiR-loaded NP and RD2-NP2.4 水迷宫行为学考察结果
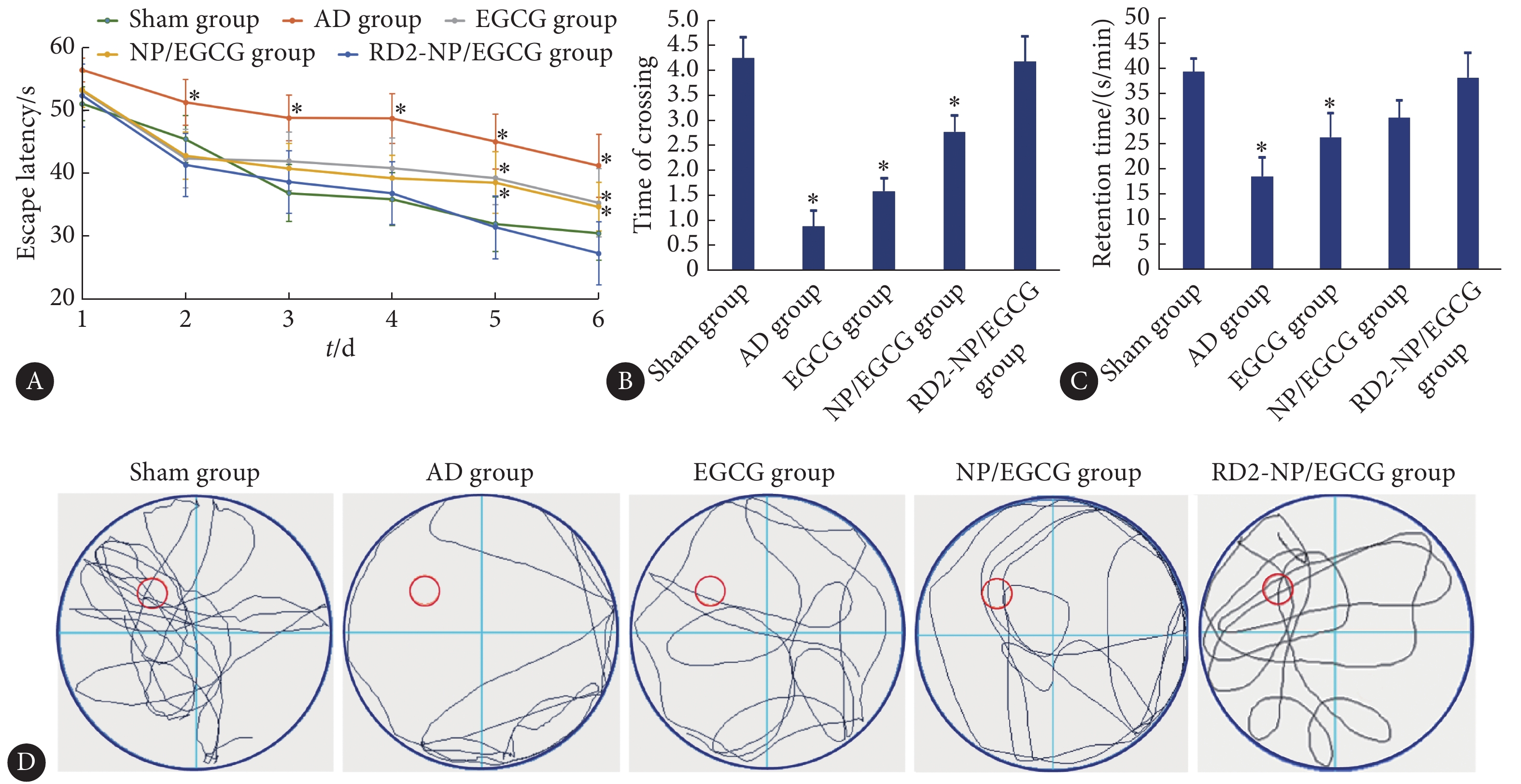
在为期6 d的训练过程中,AD对照组的潜伏期并未显著缩短(图3A),表明海马内注射Aβ42损伤了小鼠的记忆力,模型构建成功。EGCG溶液、NP/EGCG治疗4周后,AD小鼠上台潜伏期缩短,显示出一定记忆改善作用。而给予RD2-NP/EGCG治疗后,小鼠潜伏期缩短最明显,训练第2天即与AD对照组差异有统计学意义(P<0.05),且与假手术组相当。在空间探索实验(图3B~3D)中,RD2-NP/EGCG组小鼠表现出明显的寻台意识,优先在目标象限内游泳且跨越平台位置的次数与假手术组接近。而AD对照组小鼠无寻台意识,游泳轨迹主要贴着池壁边缘。这些结果表明,通过RD2-NP/EGCG的治疗可逆转AD小鼠的空间记忆障碍。
![]() 图 3 RD2-NP/EGCG改善AD模型小鼠的认知功能障碍(n=9)Figure 3. RD2-NP/EGCG ameliorated cognitive deficit in AD model mice (n=9)A: Mean escape latency in the Morris water maze of AD model mice; B and C: The times of crossing of the removed-platform area and retention time in the quadrant where the platform was placed before; D: Representative swimming path. * P<0.05, vs. RD2-NP/EGCG group.
图 3 RD2-NP/EGCG改善AD模型小鼠的认知功能障碍(n=9)Figure 3. RD2-NP/EGCG ameliorated cognitive deficit in AD model mice (n=9)A: Mean escape latency in the Morris water maze of AD model mice; B and C: The times of crossing of the removed-platform area and retention time in the quadrant where the platform was placed before; D: Representative swimming path. * P<0.05, vs. RD2-NP/EGCG group.2.5 尼氏染色观察海马区神经元形态
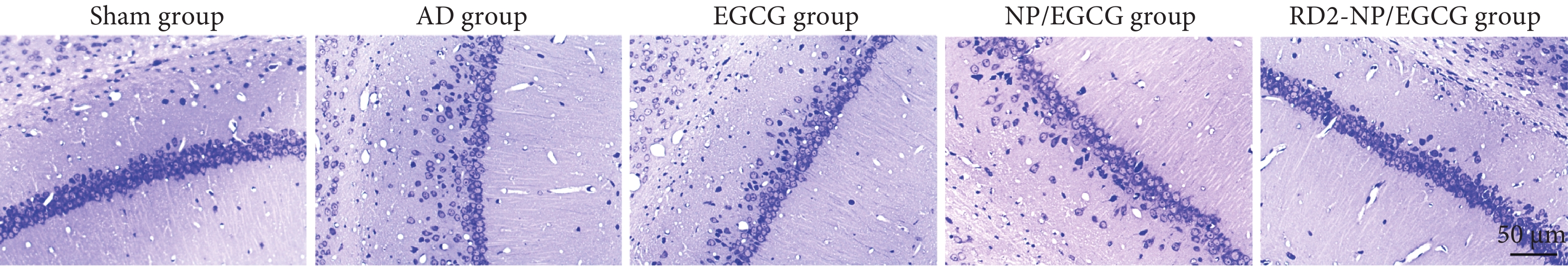
如图4所示,AD对照组小鼠CA1区有明显的神经元损伤,包括神经元丢失、排序散乱、细胞萎缩。EGCG溶液或NP/EGCG治疗后,神经元损伤得到一定改善,但仍存在神经元丢失,部分细胞皱缩、深染的现象。而RD2-NP/EGCG治疗组神经元形态正常,细胞排列密集有序,与假手术组健康的神经元相似,表明RD2-NP/EGCG显著减轻了Aβ42所致海马神经元损伤。
2.6 RD2-NP/EGCG对炎症因子表达的影响
AD小鼠海马中TNF-α和IL-1β的水平较假手术组分别提高了1.75倍和1.5倍,表明造模后小鼠海马出现严重的炎症反应(图5)。给药4周后,EGCG溶液未显著降低这两种炎性细胞因子的水平,推测与EGCG透过血脑屏障的量较少有关。RD2-NP/EGCG治疗后,小鼠海马中TNF-α和IL-1β的表达恢复至正常水平,表明RD2-NP/EGCG有效改善了AD小鼠脑部炎症微环境。
2.7 HE染色观察各脏器病理学改变
见图6。EGCG各制剂连续给药4周,心、肝、脾、肺、肾的HE染色可见各脏器结构清晰完整,无炎性细胞浸润,无异常肿胀、充血或坏死,与假手术组无明显差异。以上结果表明,RD2-NP/EGCG具有良好的体内安全性。
3. 讨论
EGCG具有广泛的药理活性,在各种神经退行性疾病中显示出治疗潜力。研究报道,EGCG能够减轻AD模型中Aβ诱导的神经元丢失[13]。口服EGCG可以明显抑制黑质中多巴胺神经元损失和帕金森病(Parkinsons’s disease, PD)模型中酪氨酸羟化酶蛋白水平的降低[14]。EGCG还可通过降低神经炎症水平和癫痫发作阈值起到抗惊厥作用[15]。但EGCG为P-糖蛋白和多药耐药相关蛋白2的底物[16],口服易被外排,吸收差,造成生物利用度低。且EGCG为多酚类化合物,在体内较易降解,稳定性较差[17]。因此,如何克服上述缺陷,充分发挥EGCG的药理活性以治疗神经退行性疾病成为亟待解决的问题。
本研究采用生物相容性好、生物可降解的PEG-PLA为材料,将EGCG包载于PEG-PLA纳米粒中,通过静注给药,大幅提高EGCG的生物利用度。同时纳米粒的包载有利于增加EGCG的稳定性,避免血脑屏障上丰富的外排蛋白对EGCG的外排作用,利于递送药物入脑。为了进一步增强纳米粒的脑转运效率和在AD病变部位的浓集,本研究在EGCG纳米粒表面修饰靶向脑淀粉斑的多肽RD2。实验结果显示,制备的RD2-NP/EGCG粒径为200 nm左右,包封率为(94.39±0.86)%。体内外研究证实RD2肽修饰赋予了纳米粒较强的Aβ结合能力,使之在AD病变区域——大脑皮质和海马的分布显著高于未修饰纳米粒。动物药效结果进一步显示,RD2-NP/EGCG在改善AD模型小鼠记忆损伤、修复神经元变性、抑制炎性反应方面均明显优于游离EGCG,且体内安全性良好。
以上结果表明本研究构建的脑淀粉斑靶向纳米递释系统具有高效递送药物到AD病变部位的能力,从而显著改善了药物对AD的治疗效果。此外,该纳米系统制备简单、生物安全性良好,适于亲脂性、亲水性药物的包载,具有潜在的应用价值。当然,主动靶向递释系统要实现临床转化,还需要进行更深入的基础研究,以及需解决产业化存在的瓶颈问题。
* * *
利益冲突 所有作者均声明不存在利益冲突
-
图 1 Th-T分析RD2-NP对Aβ42聚集的影响(n=3)
Figure 1. The influence of RD2-NP on Aβ42 aggregation was analyzed with Thioflavin T assay (n=3)
Binding to Aβ42 was determined using the Thioflavin T fluorescence at 440 nm excitation wavelength and 490 nm emission wavelength and presented in relative fluorescence units. *P<0.05, vs. RD2-NP+Aβ42 group.
图 2 离体成像观察纳米粒在脑病变部位(转基因鼠的海马和大脑皮质)的分布和相对荧光强度
A: Ex vivo imaging of hippocampus and cerebral cortex; B: Relative fluorescence intensity of hippocampus and cerebral cortex from each mice group (n=3). **P<0.01.
Figure 2. The ex vivo imaging of brain lesions of 3×Tg-AD transgenic mice at 1 h after intravenous injection of DiR-loaded NP and RD2-NP
图 3 RD2-NP/EGCG改善AD模型小鼠的认知功能障碍(n=9)
Figure 3. RD2-NP/EGCG ameliorated cognitive deficit in AD model mice (n=9)
A: Mean escape latency in the Morris water maze of AD model mice; B and C: The times of crossing of the removed-platform area and retention time in the quadrant where the platform was placed before; D: Representative swimming path. * P<0.05, vs. RD2-NP/EGCG group.
表 1 不同纳米粒的理化性质(n=3)
Table 1 Physicochemical characterization of different nanoparticles (n=3)
Nanoparticles Size/nm PDI Zeta potential/mV DLC/% EE/% NP/EGCG 198.54±3.67 0.185±0.042 −23.51±0.32 95.43±1.02 5.96±0.06 RD2-NP/EGCG 204.83±2.80 0.191±0.066 −23.88±1.24 94.39±0.86 5.90±0.05 PDI: Polydispersity index; DLC: Drug loading capacity; EE: Encapsulation efficiency. -
[1] PLASSMAN B L, LANGA K M, FISHER G G, et al. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology,2007,29(1/2): 125–132. DOI: 10.1159/000109998
[2] FRISONI G B, BOCCARDI M, BARKHOF F, et al. Strategic roadmap for an early diagnosis of Alzheimer’s disease based on biomarkers. Lancet Neurol,2017,16(8): 661–676. DOI: 10.1016/S1474-4422(17)30159-X
[3] WONG K H, RIAZ M K, XIE Y, et al. Review of current strategies for delivering Alzheimer's disease drugs across the blood-brain barrier. Int J Mol Sci,2019,20(2): 381–406. DOI: 10.3390/ijms20020381
[4] YANG P, SHENG D, GUO Q, et al. Neuronal mitochondria-targeted micelles relieving oxidative stress for delayed progression of Alzheimer's disease. Biomaterials,2020,238: 1–12. DOI: 10.1016/j.biomaterials.2020.119844
[5] GUO Q, ZHENG X, YANG P, et al. Small interfering RNA delivery to the neurons near the amyloid plaques for improved treatment of Alzheimer’s disease. Acta Pharm Sin B,2019,9(3): 590–603. DOI: 10.1016/j.apsb.2018.12.010
[6] CARTER M D, SIMMS G A, WEAVER D F. The development of new therapeutics for Alzheimer’s disease. Clin Pharmacol Ther,2010,88(4): 475–486. DOI: 10.1038/clpt.2010.165
[7] VAN GROEN T, SCHEMMERT S, BRENER O, et al. The Abeta oligomer eliminating D-enantiomeric peptide RD2 improves cognition without changing plaque pathology. Sci Rep,2017,7(1): 16275. DOI: 10.1038/s41598-017-16565-1
[8] GRANJA A, FRIAS I, NEVES A R, et al. Therapeutic potential of Epigallocatechin Gallate nanodelivery systems. Biomed Res Int,2017,17: 1–15. DOI: 10.1155/2017/5813793
[9] ZHANG C, WAN X, ZHENG X Y, et al. Dual-functional nanoparticles targeting amyloid plaques in the brains of Alzheimer’s disease mice. Biomaterials,2014,35: 456–465. DOI: 10.1016/j.biomaterials.2013.09.063
[10] SINGH N A, BHARDWAJ V, RAVI C, et al. EGCG nanoparticles attenuate aluminum chloride induced neurobehavioral deficits, beta amyloid and tau pathology in a rat model of Alzheimer’s disease. Front Aging Neurosci,2018,10: 244[2021-03-01]. https://doi.org/10.3389/fnagi.2018.00244.
[11] ZHANG C, ZHENG X Y, WAN X, et al. The potential use of H102 peptide-loaded dual-functional nanoparticles in the treatment of Alzheimer’s disease. J Control Release,2014,192: 317–324. DOI: 10.1016/j.jconrel.2014.07.050
[12] GUO Q, XU S, YANG P, et al. A dual-ligand fusion peptide improves the brain-neuron targeting of nanocarriers in Alzheimer’s disease mice. J Control Release,2020,320: 347–362. DOI: 10.1016/j.jconrel.2020.01.039
[13] KAUR T, PATHAK C M, PANDHI P, et al. Effects of green tea extract on learning, memory, behavior and acetylcholinesterase activity in young and old male rats. Brain Cogn,2008,67(1): 25–30. DOI: 10.1016/j.bandc.2007.10.003
[14] LEVITES Y, WEINREB O, MAOR G, et al. Green tea polyphenol (−)-Epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. J Neurochem,2010,78(5): 1073–1082. DOI: 10.1046/j.1471-4159.2001.00490.x
[15] CANO A, ETTCHETO M, ESPINA M, et al. Epigallocatechin-3-gallate loaded PEGylated-PLGA nanoparticles: A new anti-seizure strategy for temporal lobe epilepsy. Nanomedicine,2018,14(4): 1073–1085. DOI: 10.1016/j.nano.2018.01.019
[16] 郭子涛. 表没食子儿茶素没食子酸酯(EGCG)在Caco-2细胞中的摄取、跨膜转运和外排研究. 上海: 华东师范大学, 2011. [17] KRUPKOVA O, FERGUSON S J, WUERTZ-KOZAK K. Stability of (−)-epigallocatechin gallate and its activity in liquid formulations and delivery systems. J Nutr Biochem,2016,37: 1–12. DOI: 10.1016/j.jnutbio.2016.01.002
-
期刊类型引用(3)
1. 肖湘芝,陈管雄,胡智文. 表没食子儿茶素没食子酸酯靶向调控Nrf2-Keap1信号通路与脑梗死神经保护作用的关系. 实用医学杂志. 2024(03): 309-315 .  百度学术
百度学术
2. 郎尉雅,张可爽,张晨曦,李林,张海燕. 基于网络药理学探讨表没食子儿茶素没食子酸酯治疗阿尔茨海默病的作用机制. 中国当代医药. 2023(32): 9-13+198 .  百度学术
百度学术
3. 唐静怡,周方,曾鸿哲,李秀平,欧阳建,黄建安,刘仲华. 茶叶功能成分预防阿尔茨海默病研究进展. 食品工业科技. 2022(17): 501-513 .  百度学术
百度学术
其他类型引用(0)

 首页
首页


 下载:
下载: