Effect of Connexin on Cochlear Blood-Labyrinth Barrier in a Mouse Model of Endolymphatic Hydrops
-
摘要:目的 本研究拟采用自发性膜迷路积水动物模型PHEX基因突变小鼠研究紧密连接蛋白ZO-1在耳蜗血管纹组织中的表达,分析突变体小鼠在病理学、影像学及听力功能上的动态改变。方法 选取出生后21 d(P21)、出生后90 d(P90)、出生后120 d(P120)的PHEX基因突变的Hyp-Duk/Y雄性小鼠为实验组,同龄的野生型雄性小鼠为对照组,耳蜗切片HE染色观察有无膜迷路积水表现并评估严重程度,取耳蜗行免疫组化染色评估紧密连接蛋白ZO-1的表达;两组均在P90时检测听觉诱发脑干反应(auditory-evoked brainstem response, ABR),在P21、P90、P120行7.0 T MRI钆造影活体观察耳蜗中阶内淋巴扩张情况。结果 P90、P120的实验组小鼠病理切片HE染色可见膜迷路积水,血管纹ZO-1水平较同龄对照组降低,差异有统计学意义(P<0.05)。ZO-1表达水平与膜迷路积水程度呈明显负相关(r=−0.939,P<0.01)。P90的实验组小鼠双侧ABR阈值较同日龄对照组升高,实验组小鼠双侧呈不对称性听力下降。P90、P120的实验组小鼠通过MRI钆造影可活体观察到重度膜迷路积水改变。结论 PHEX Hyp-Duk/Y可作为梅尼埃病基础研究的良好模型。PHEX Hyp-Duk/Y小鼠发生膜迷路积水的机制与紧密连接蛋白ZO-1表达水平下降导致血管纹血迷路屏障功能破坏有关。7.0 T MRI钆造影可活体观察小鼠重度膜迷路积水改变,为梅尼埃病的诊断提供影像学依据。Abstract:Objective To examine the expression of tight-junction connexin ZO-1 in the stria vascularis tissue of the cochlea by using spontaneous endolymphatic hydrops animal model constructed with PHEX gene mutant mice, and to analyze the dynamic changes of the gene mutant mice in pathology, imaging, and hearing function.Methods Male Hyp-Duk/Y mice with PHEX gene mutation were selected as the experimental group at three time points, 21 days post birth (P21), 90 days post birth (P90) and 120 days post birth (P120), and wild-type male mice of the same ages were selected as the control groups. The cochlear sections were HE-stained in order to observe whether endolymphatic hydrops was present or absent and to assess its severity. The expression of connexin ZO-1 in both groups was evaluated through immunohistochemical staining of cochlear sections. Auditory-evoked brainstem response (ABR) was induced in both groups at P90 and gadolinium-enhanced MRI was conducted in vivo to observe the middle-order endolymphatic dilatation of cochlea in experimental and control mice aged P21, P90 and P120.Results HE staining of pathological sections of PHEX Hyp-Duk/Y mice aged P90 and P120 showed increased endolymphatic hydronephrosis. The level of striae ZO-1 in PHEX Hyp-Duk/Y mice aged P90 and P120 was significantly lower than that of the controls of the same age (P<0.05). The expression level of ZO-1 was significantly negatively correlated with the degree of endolymphatic hydronephrosis (r=−0.939, P<0.01). The bilateral ABR threshold of PHEX Hyp-Duk/Y mice aged P90 was higher than that of the wild-type mice of the same age, and the mutant mice showed asymmetric hearing loss on both sides. Severe endolymphatic hydronephrosis was observed in PHEX Hyp-Duk/Y mice aged P90 and P120 through in vivo MRI gadolinium imaging.Conclusion PHEX Hyp-Duk/Y can be used as a sound model for basic research of Ménière’s disease. Compared with wild-type mice, PHEX Hyp-Duk/Y mice showed decreased expression of connexin protein ZO-1, which damaged the function of the blood-labyrinth barrier in stria vascularis, and was involved in the formation of endolymphatic hydrops. 7.0 T MRI gadolinium imaging can be used to observe the changes of severe endolymphatic hydrops in mice in vivo, providing imaging basis for the diagnosis of Ménière’s disease.
-
Keywords:
- PHEX gene mouse /
- Endolymphatic hydrops /
- Blood-labyrinth barrier /
- Hearing loss /
- Ménière’s disease /
- MRI
-
梅尼埃病(Ménière’s disease, MD)病理特征为膜迷路积水,主要表现在蜗管和球囊积水。MD存在多种病因假说,包括血-迷路屏障破坏、内淋巴清除率降低、内淋巴分泌紊乱、变态反应等[1]。血-迷路屏障位于血管纹边缘细胞和基底细胞之间,除了内皮细胞和基底膜外,还包含周细胞及血管旁巨噬细胞共同组成血-迷路屏障[2-4],各细胞之间以不同形式的细胞连接相连,而血管纹内皮细胞的通透性与紧密连接蛋白ZO-1的分布表达密切相关,表达下降时血管通透性增加[5]。MD患者无法行病理检查,随着磁共振内耳造影技术的发展,内耳内淋巴显像对于临床诊断MD具有重要意义。本研究以能自发产生膜迷路积水和进行性听力下降的PHEX Hyp-Duk/Y小鼠[6]为实验对象,探索膜迷路积水模型血管纹连接蛋白的改变引起膜迷路积水的发病机制,以及MRI在膜迷路积水诊断中的运用。
1. 材料与方法
1.1 动物
PHEX Hyp-Duk基因小鼠购于美国Jackson实验室,经繁殖后选择日龄为21日、90日和120日(P21、P90和P120)的小鼠,并采用PCR鉴定出突变型PHEX Hyp-Duk/Y雄性小鼠(实验组)和野生型雄性小鼠(对照组)。
1.2 试剂与仪器
兔多克隆抗体Anti ZO-1购自Invitrogen公司;正置荧光显微镜(Zeiss公司,型号AX10 imager A2/AX10 cam HRC);体式显微镜(Zeiss公司,型号Discovery.V8);超薄切片机(Zeiss公司,型号EM Uc6v);小动物7.0 T磁共振成像系统(Bruker公司,型号BioSpec70/30);Navigator Pro Auditory Evoked Potential AEP System (Biologic公司)。
1.3 方法
1.3.1 HE染色和ZO-1蛋白的免疫组化染色
HE染色:实验组和对照组各12只,选取日龄为P21、P90和P120的小鼠,经麻醉满意后,采用磷酸盐缓冲液(PBS)及质量分数4%中性多聚甲醛行快速心脏灌注,随即断头后取出耳蜗,放置于质量分数4%中性多聚甲醛中固定,4 ℃过夜;换用10%乙二酸四乙酸(EDTA)浸泡耳蜗标本进行脱钙,以切面与耳蜗蜗轴平行的方向包埋;冻台静置30 min后切片,切片厚度5 μm;小鼠耳蜗组织石蜡切片65 ℃烤片过夜;苏木素染色,伊红染色;流水冲洗切片,梯度浓度乙醇脱水,37 ℃烘箱内烤片至无液体残留后中性树胶进行封片。
免疫组化染色:同法选取P21、P90、P120的实验组(15只)和对照组(13只)的耳蜗组织蜡块切片,厚度5 μm,65 ℃烤片过夜;二甲苯溶液、梯度浓度乙醇溶液浸泡,PBS清洗,放入0.01 mmol/L枸橼酸钠溶液浸泡40 min进行抗原修复;5%山羊血清溶液封闭,加入一抗工作液(兔多克隆抗体Anti ZO-1, 1∶100)4 ℃冰箱过夜;PBS清洗后滴加羊抗兔二抗工作液; PBS清洗,二氨基联苯胺染色液显色,再次PBS冲洗10~20 min;脱水后封片。
1.3.2 病理切片判定
淋巴积水判定:使用从0到4的等级划分病理内淋巴积水严重程度[7]。 0级:以野生型前庭膜位置为正常;无膜迷路积水;1级:前庭膜轻微膨隆;2级:前庭膜隆起与临近血管纹的前庭阶内壁相接;3级:前庭膜明显膨隆,一半以上与前庭阶顶壁接触;4级:内淋巴积水占前庭阶、中阶总面积的75%以上。此判定采用HE染色切片。
膜迷路积水程度判定:使用Image J分析软件获取实验组小鼠各年龄段HE切片的中阶、前庭阶面积,计算中阶面积占总面积的比值(总面积为中阶面积与前庭阶面积之和),此比值越大,膜迷路积水程度越重。此判定采用HE染色切片。
ZO-1表达水平判定:使用Image J分析软件获取P21、P90、P120实验组与对照组血管纹组织免疫组化图像ZO-1表达水平灰度值并计算各组灰度值平均值。此判定采用免疫组化染色切片。
1.3.3 小鼠听觉诱发脑干反应(auditory-evoked brainstem response, ABR)检测听力阈值
选取P90的实验组和对照组小鼠各3只,麻醉满意后,俯卧位固定,记录电极插入小鼠两耳廓连线正中皮下,参考电极朝向腮部插入,分别插入两侧耳垂皮下;测试隔音屏蔽室内进行避免环境因素干扰,采用Navigator Pro Auditory Evoked Potential AEP System Bio logic, USA设备及系统进行测试,使用短声Click作为刺激音。
1.3.4 7.0 T MRI观测耳蜗膜迷路改变
在P21、P90、P120三个时间点,选择实验组和对照组小鼠各4只;采用钆增强进行内耳磁共振薄层扫描。提前半小时经腹腔注射469.01 mg/mL造影剂钆喷酸葡胺注射液100 μL。将小鼠放置于密闭容器中,异氟烷诱导麻醉满意后将小鼠固定于操作台上,调整体位使小鼠头部位于正中无偏斜,胶带固定小鼠背部,减少呼吸动度的影响。采用自旋回波序列,扫描参数为重复时间100 ms,回波时间2.5 ms,采集过程中异氟烷维持麻醉,并监测小鼠呼吸。NAKASHIMA等[8]将耳蜗膜迷路积水分为3级:无膜迷路积水:前庭膜无位移;轻度膜迷路积水:前庭膜位移但中阶面积小于前庭阶;重度膜迷路积水:中阶面积超过前庭阶。采用此方法定性评估膜迷路积水改变。使用Image J分析软件在薄层磁共成像基础上测量耳蜗中阶面积、前庭阶面积。计算中阶面积占总面积的比值(总面积为中阶面积与前庭阶面积之和),进一步定量评价膜迷路积水严重程度。
1.3.5 统计学方法
计量资料均以
$ \bar x \pm s $ 表示,组间均值比较采用非配对t检验。对紧密连接蛋白ZO-1表达水平灰度值数据与膜迷路积水严重程度(HE切片中阶面积总面积比值)关系进行Pearson相关性分析。 P<0.05为差异有统计学意义。2. 结果
2.1 耳蜗切片HE染色结果
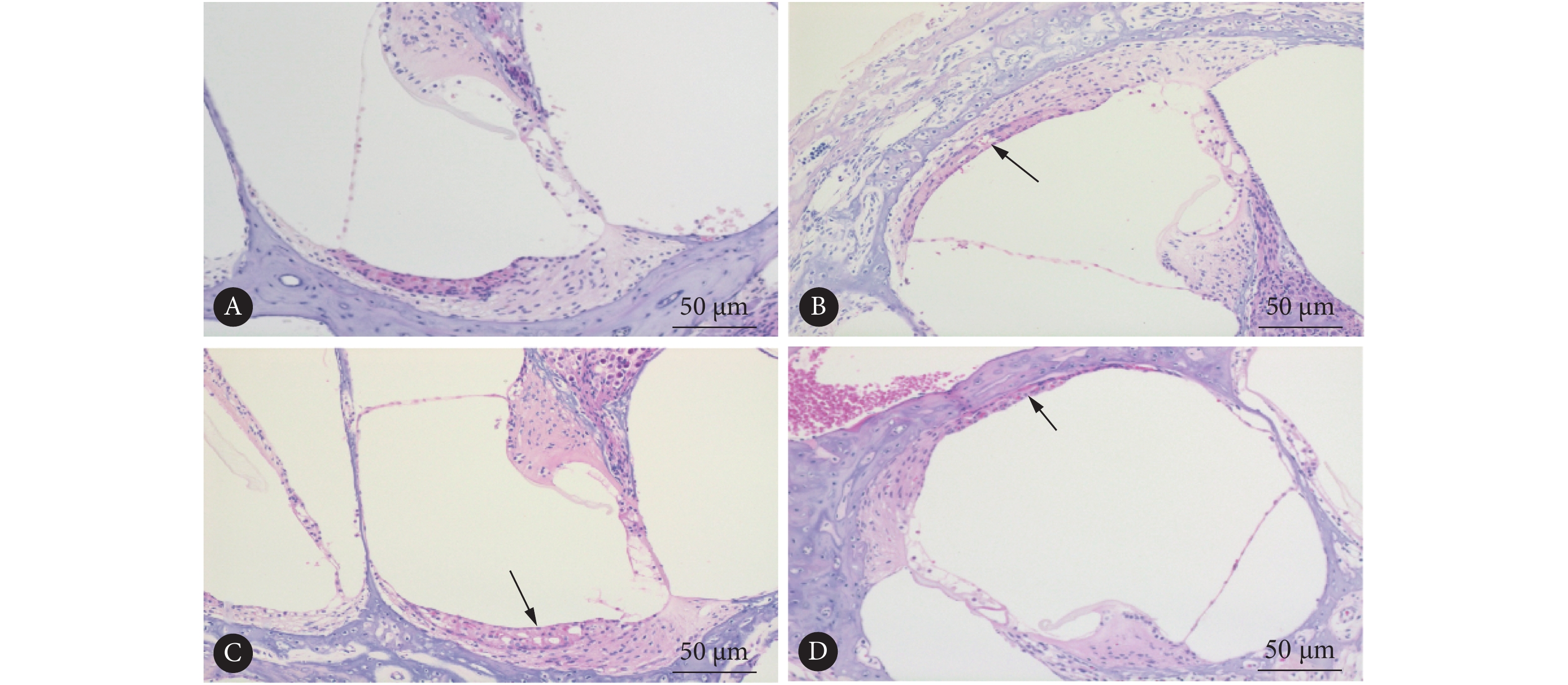
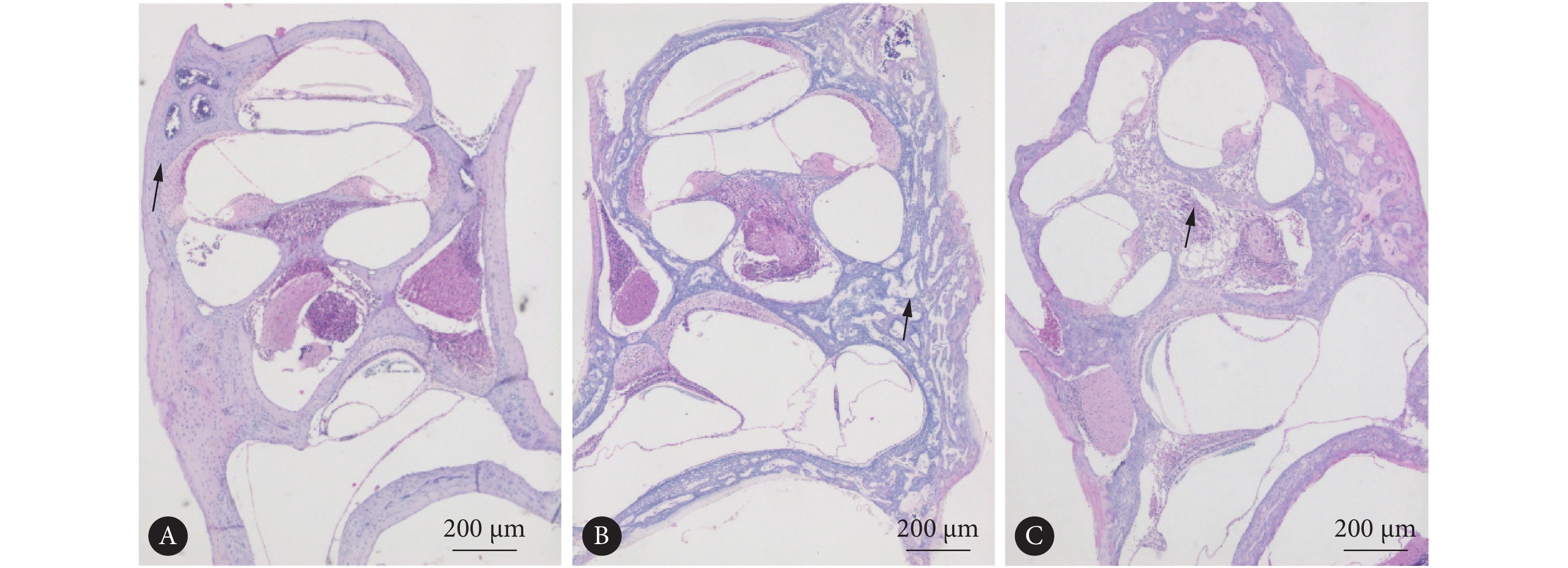
对照组小鼠前庭膜处于正常位置,骨化良好(图1A)。而所有实验组均可见成骨细胞排列紊乱,骨化不全(图1B)。在年龄为P120的实验组小鼠切片中,可见螺旋神经节变性、排列紊乱(图1C)。在实验组 P90、P120小鼠切片中可见前庭膜隆起、中阶扩张等膜迷路积水改变(图1B、1C)。
![]() 图 1 实验组和对照组耳蜗HE染色观察淋巴积水Figure 1. Observing lymphatic hydrops in HE-stained cochlea sections of the experimental group and the control groupA: P90 control group showed good ossification; B: P90 experimental group, with the arrow indicating disordered osteoblasts and incomplete ossification, and grade 3 endolymphatic hydropand vestibular membrane swelling was observed in the cochlear duct; C: P120 experimental group showed grade 4 endolymphatic hydrops and serious vestibular membrane swelling, with the arrow indicating degeneration of spiral ganglion.
图 1 实验组和对照组耳蜗HE染色观察淋巴积水Figure 1. Observing lymphatic hydrops in HE-stained cochlea sections of the experimental group and the control groupA: P90 control group showed good ossification; B: P90 experimental group, with the arrow indicating disordered osteoblasts and incomplete ossification, and grade 3 endolymphatic hydropand vestibular membrane swelling was observed in the cochlear duct; C: P120 experimental group showed grade 4 endolymphatic hydrops and serious vestibular membrane swelling, with the arrow indicating degeneration of spiral ganglion.对照组P90小鼠无前庭膜隆起、中阶扩张,骨化良好(图2A),实验组P21小鼠无前庭膜隆起、中阶扩张等膜迷路积水改变(图2B);而实验组P90、P120小鼠耳蜗HE切片中均可见到不同程度的膜迷路积水(图2C、2D),且血管纹切面厚度较野生型减小(图2C),在实验组小鼠无膜迷路积水及3级膜迷路积水时可见血管纹空泡形成(图2B、2C)。随着日龄增加,P21(40.91%±3.21%)、P90(72.27%±3.91%)、P120实验组小鼠(81.89%±8.20%)HE染色切片中阶面积占总面积比值逐渐增加(P<0.05),反映膜迷路积水程度逐渐加重。
![]() 图 2 对照组与实验组耳蜗HE染色观察膜迷路积水Figure 2. Observing endolymphatic hydrops in HE-stained cochlea sections of the control group and the experimental groupA: Control group showed normal cochlear duct; B: P21 experimental group, with the arrow showing vacuolar formation in stria vascularis; C: P90 experimental group showed grade 3 endolymphatic hydrops, and the arrow displayed vesicular formation in stria vascularis; D: P120 experimental group showed grade 4 endolymphatic hydrops, and the arrow displayed decreased thickness of stria vascularis.
图 2 对照组与实验组耳蜗HE染色观察膜迷路积水Figure 2. Observing endolymphatic hydrops in HE-stained cochlea sections of the control group and the experimental groupA: Control group showed normal cochlear duct; B: P21 experimental group, with the arrow showing vacuolar formation in stria vascularis; C: P90 experimental group showed grade 3 endolymphatic hydrops, and the arrow displayed vesicular formation in stria vascularis; D: P120 experimental group showed grade 4 endolymphatic hydrops, and the arrow displayed decreased thickness of stria vascularis.2.2 耳蜗血管纹组织经免疫组化染色检测连接蛋白ZO-1的表达
实验组P21小鼠与对照组小鼠血管纹紧密连接蛋白ZO-1表达水平无明显差异;在P90、P120时,实验组耳蜗血管纹ZO-1表达水平均低于同日龄对照组(P<0.01,图3A,表1)。通过单因素方差分析,不同日龄实验组ZO-1的表达水平差异有统计学意义(P=0.001,F=20.55,表1),进一步两两对比分析,P21与P90、P120蛋白表达对比差异均有统计学意义(P=0.001),但P90与P120之间ZO-1表达无明显差异(P>0.05)。将实验组ZO-1表达水平与HE切片中阶面积占总面积比值进行Pearson相关性分析,蛋白表达下降越严重,膜迷路积水程度越严重,两者间存在相关性,ZO-1表达水平与膜迷路积水程度呈明显负相关(r=−0.939,P<0.01)。
![]() 图 3 耳蜗血管纹组织经免疫组化染色检测ZO-1的表达(A)和内耳钆造影MRI(B)Figure 3. Immunohistochemical staining of the cochlear stria vascularis to examine the expression of connexin ZO-1 (A) and gadolinium-enhanced MRI of inner ear (B)The gadolinium-enhanced MRI of inner ear showed that the high signal was perilympha and the low signal was endolymph; a-c and e: there was no change of cochlear duct in any control groups or P21 experimental group; d and f: the low signals shown by the arrows were severe endolymphatic hydrops, and the area of high signal in vestibular stage was significantly reduced and smaller than the area of cochlear duct.表 1 实验组与对照组免疫组化染色ZO-1表达水平及MRI中阶面积占总面积比值Table 1. Gray values of ZO-1 expression and ratio of cochlear duct area to total area in MRI in the experimental groups and the control groups
图 3 耳蜗血管纹组织经免疫组化染色检测ZO-1的表达(A)和内耳钆造影MRI(B)Figure 3. Immunohistochemical staining of the cochlear stria vascularis to examine the expression of connexin ZO-1 (A) and gadolinium-enhanced MRI of inner ear (B)The gadolinium-enhanced MRI of inner ear showed that the high signal was perilympha and the low signal was endolymph; a-c and e: there was no change of cochlear duct in any control groups or P21 experimental group; d and f: the low signals shown by the arrows were severe endolymphatic hydrops, and the area of high signal in vestibular stage was significantly reduced and smaller than the area of cochlear duct.表 1 实验组与对照组免疫组化染色ZO-1表达水平及MRI中阶面积占总面积比值Table 1. Gray values of ZO-1 expression and ratio of cochlear duct area to total area in MRI in the experimental groups and the control groupsAge Group n ZO-1 expression n Ratio of cochlear duct area to total area/% P21 Control 5 62.08±4.72 4 25.13±5.09 Experimental 4 60.40±6.67 4 26.48±8.80 P90 Control 4 60.76±9.27 4 26.56±4.89 Experimental 7 35.80±7.33*,# 4 63.37±2.71# P120 Control 4 56.27±9.65 4 26.00±3.31 Experimental 4 24.25±4.47*, # 4 67.47±5.83# # P<0.05, vs. control group at the same age; * P=0.001, vs. P21 experimental group. 2.3 PHEX基因突变小鼠ABR检测听力阈值升高
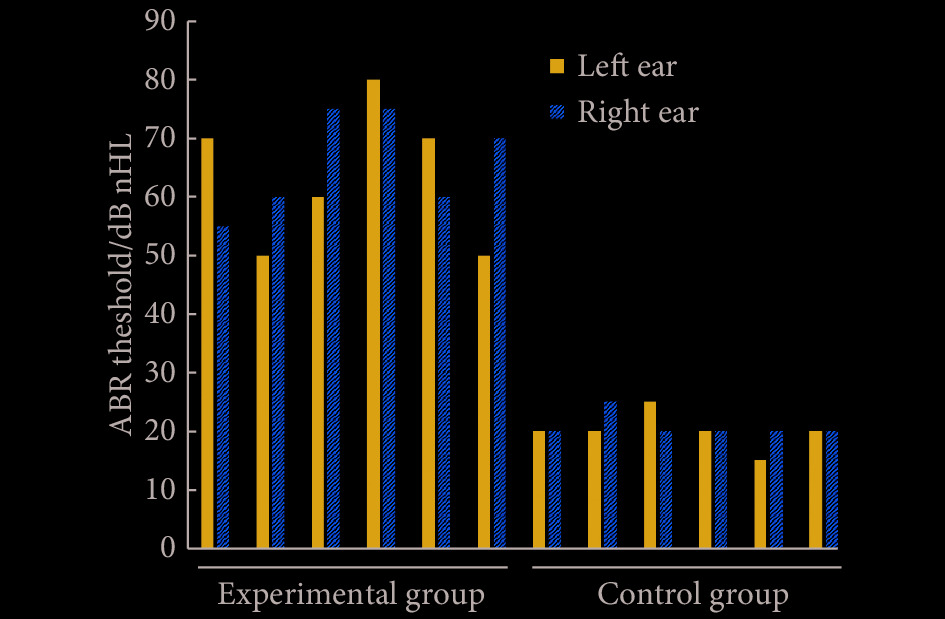
对P90的突变小鼠及野生型小鼠以click作为声刺激时,可见突变小鼠反应阈值高于野生型小鼠,突变体小鼠左右耳呈不对称性听力下降(图4)。
2.4 钆增强造影MRI内耳观察结果
增强MRI示,P21实验组、对照组均无膜迷路积水表现(图3Ba~3Bc,3Be),P21实验组与对照组中阶面积与总面积比值无明显差异(P>0.05,表1);P90、P120 PHEX Hyp-Duk/Y小鼠膜迷路积水MRI分级均为3级(图3Bd、3Bf),P90、P120 PHEX Hyp-Duk/Y小鼠中阶面积均明显大于前庭阶;实验组与对照组中阶面积与总面积比值差异有统计学意义(P<0.05,表1)。
3. 讨论
PHEX基因是位于X染色体上的磷酸调节基因,PHEX的等位突变基因体PHEX Hyp-Duk/Y小鼠出生后出现自发性膜迷路积水及进行性加重的听力障碍。但并非所有PHEX Hyp-Duk/+雌性动物都显示出一致的内耳表型,而雄性则常表现出稳定的内耳表型(自发性膜迷路积水及听力损失)[6],故本研究选择PHEX Hyp-Duk/Y和野生型雄性小鼠作为实验组及对照组进行研究。
文献中报道PHEX Hyp-Duk/Y小鼠在P15开始出现前庭症状;P21~P30出现听力损失发作,非对称性发作进行性加重;P25才出现顶旋膜迷路积水,ABR和组织学数据表明,功能性变性先于结构性变性,后期膜迷路积水导致螺旋神经节细胞和毛细胞死亡[6, 9]。本实验发现实验组小鼠在P120时膜迷路积水最为严重。
紧密连接(TJs)存在于内皮细胞之间、基底细胞之间和边缘细胞之间。TJs蛋白在耳蜗毛细血管内皮细胞中高浓度表达,构成毛细血管选择性通透的功能基础[10]。紧密连接蛋白ZO-1与内皮细胞的通透性密切相关,常作为观察屏障功能和通透性的指标[11]。本实验发现实验组小鼠膜迷路积水在P120时最为严重,ZO-1蛋白表达和积水严重程度呈明显负相关,提示ZO-1表达下降导致血-迷路屏障破坏、血管通透性增加,导致膜迷路积水的发生,蛋白表达下降越严重,膜迷路积水程度越严重。
MELKI等[7]研究发现实验组小鼠听力损失的严重程度与膜迷路积水的严重程度相关。人类MD研究中通过MRI也发现听力下降程度与膜迷路积水程度相关[12],进一步说明了该小鼠是较为接近MD的动物模型。多数研究中MRI观察活体膜迷路积水动物模型均使用豚鼠,DEGERMAN成功运用9.4 T MRI活体观察到药物诱导膜迷路积水小鼠内淋巴扩张表现[13]。本研究采用7.0 T MRI自旋回波序列可成功观察小鼠重度膜迷路积水,但在扫描图像上P90 、P120未见明显差异。实验组缺少轻度膜迷路积水的小鼠,7.0 T MRI对小鼠轻度膜迷路积水的诊断效果有待进一步的研究。动物模型中钆造影剂途径多采用腹腔注射、静脉注射或经鼓膜注射,经鼓膜注射容易损伤内耳结构,造成实验误差。本实验采用腹腔注射,相比鼓膜注射创伤更小、更安全。注射后造影剂随循环到达内耳,但由于正常血-迷路屏障存在,造影剂只能到达鼓阶及前庭阶外淋巴,而无法进入耳蜗中阶内淋巴腔隙,缩短了外淋巴液的弛豫时间,使其在MRI上增强显影,从而达到区分内外淋巴的效果,而当血-迷路屏障破坏时,造影剂则会渗入内淋巴液中;但本次实验中未能观察到明显造影剂泄露至内淋巴液,可能是扫描时间较短的缘故。此外,临床检查中还多采用三维液体衰减反转恢复(three-dimensional fluid attenuated inversion recovery, 3D FLAIR)扫描序列抑制内淋巴液的信号,使得外淋巴间隙呈高信号影,内淋巴液为低信号影,更好的区别内外淋巴[14],以后可尝试作为弥补手段。
本研究已发现紧密连接蛋白ZO-1表达水平参与膜迷路积水形成,但PHEX基因突变导致ZO-1表达水平变化的调控机制仍需进一步研究,ZO-1表达下调引起血迷路屏障功能受损并最终导致膜迷路积水的病理机制也有待深入探讨。
* * *
利益冲突 所有作者均声明不存在利益冲突
-
图 1 实验组和对照组耳蜗HE染色观察淋巴积水
Figure 1. Observing lymphatic hydrops in HE-stained cochlea sections of the experimental group and the control group
A: P90 control group showed good ossification; B: P90 experimental group, with the arrow indicating disordered osteoblasts and incomplete ossification, and grade 3 endolymphatic hydropand vestibular membrane swelling was observed in the cochlear duct; C: P120 experimental group showed grade 4 endolymphatic hydrops and serious vestibular membrane swelling, with the arrow indicating degeneration of spiral ganglion.
图 2 对照组与实验组耳蜗HE染色观察膜迷路积水
Figure 2. Observing endolymphatic hydrops in HE-stained cochlea sections of the control group and the experimental group
A: Control group showed normal cochlear duct; B: P21 experimental group, with the arrow showing vacuolar formation in stria vascularis; C: P90 experimental group showed grade 3 endolymphatic hydrops, and the arrow displayed vesicular formation in stria vascularis; D: P120 experimental group showed grade 4 endolymphatic hydrops, and the arrow displayed decreased thickness of stria vascularis.
图 3 耳蜗血管纹组织经免疫组化染色检测ZO-1的表达(A)和内耳钆造影MRI(B)
Figure 3. Immunohistochemical staining of the cochlear stria vascularis to examine the expression of connexin ZO-1 (A) and gadolinium-enhanced MRI of inner ear (B)
The gadolinium-enhanced MRI of inner ear showed that the high signal was perilympha and the low signal was endolymph; a-c and e: there was no change of cochlear duct in any control groups or P21 experimental group; d and f: the low signals shown by the arrows were severe endolymphatic hydrops, and the area of high signal in vestibular stage was significantly reduced and smaller than the area of cochlear duct.
表 1 实验组与对照组免疫组化染色ZO-1表达水平及MRI中阶面积占总面积比值
Table 1 Gray values of ZO-1 expression and ratio of cochlear duct area to total area in MRI in the experimental groups and the control groups
Age Group n ZO-1 expression n Ratio of cochlear duct area to total area/% P21 Control 5 62.08±4.72 4 25.13±5.09 Experimental 4 60.40±6.67 4 26.48±8.80 P90 Control 4 60.76±9.27 4 26.56±4.89 Experimental 7 35.80±7.33*,# 4 63.37±2.71# P120 Control 4 56.27±9.65 4 26.00±3.31 Experimental 4 24.25±4.47*, # 4 67.47±5.83# # P<0.05, vs. control group at the same age; * P=0.001, vs. P21 experimental group. -
[1] OBERMAN B S, PATEL V A, CUREOGLU S, et al. The aetiopathologies of Meniere’s disease: A contemporary review. Acta Otorhinolaryngol Ital,2017,37(4): 250–263. DOI: 10.14639/0392-100X-793
[2] 张桐, 韩维举. 耳蜗血管纹血-迷路屏障病理生理学研究进展. 中华耳科学杂志,2017,15(2): 257–262. DOI: 10.3969/j.issn.1672-2922.2017.02.022 [3] 江英, 姚红兵, 陈俊宏, 等. 双光子荧光显微镜结合Imaris 3D渲染技术显示耳蜗血管纹血迷路屏障细胞分布. 临床耳鼻咽喉头颈外科杂志,2020,34(6): 486–491. [4] JIANG Y, ZHANG J, RAO Y, et al. Lipopolysaccharide disrupts the cochlear blood-labyrinth barrier by activating perivascular resident macrophages and up-regulating MMP-9. Int J Pediatr Otorhinolaryngol, 2019, 127: 109656[2021-04-21]. https://doi.org/10.1016/j.ijporl.2019.109656.
[5] ZHOU W, SHI G, BAI J, et al. Colquhounia root tablet protects rat pulmonary microvascular endothelial cells against TNF-alpha-induced injury by upregulating the expression of tight junction proteins Claudin-5 and ZO-1. Evid Based Complement Alternat Med, 2018, 2018: 1024634[2021-04-21]. https://doi.org/10.1155/2018/1024634.
[6] WICK C C, SEMAAN M T, ZHENG Q Y, et al. A genetic murine model of endolymphatic hydrops: The phex mouse. Curr Otorhinolaryngol Rep,2014,2(3): 144–151. DOI: 10.1007/s40136-014-0048-7
[7] MELKI S J, LI Y, SEMAAN M T, et al. A mouse model validates the utility of electrocochleography in verifying endolymphatic hydrops. J Assoc Res Otolaryngol,2014,15(3): 413–421. DOI: 10.1007/s10162-014-0445-0
[8] NAKASHIMA T, NAGANAWA S, PYYKKO I, et al. Grading of endolymphatic hydrops using magnetic resonance imaging. Acta Otolaryngol Suppl,2009,560: 5–8. DOI: 10.1080/00016480902729827
[9] SEMAAN M T, ZHENG Q Y, HAN F, et al. Characterization of neuronal cell death in the spiral ganglia of a mouse model of endolymphatic hydrops. Otol Neurotol,2013,34(3): 559–569. DOI: 10.1097/MAO.0b013e3182868312
[10] 邓双, 董波, 徐少然, 等. C57BL/6J小鼠衰老耳蜗血管纹微血管周细胞对内皮细胞通透性影响的研究. 中华耳鼻咽喉头颈外科杂志,2021,56(11): 1185–1193. [11] ZHENG D, ZHANG J, ZHANG Z, et al. Endothelial microvesicles induce pulmonary vascular leakage and lung injury during sepsis. Front Cell Dev Biol, 2020, 8: 643[2021-04-21]. https://doi.org/10.3389/fcell.2020.00643.
[12] CHO Y S, AHN J M, CHOI J E, et al. Usefulness of intravenous gadolinium inner ear mr imaging in diagnosis of Meniere’s disease. Sci Rep,2018,8(1): 17562. DOI: 10.1038/s41598-018-35709-5
[13] DEGERMAN E, IN’T ZANDT R, PALBRINK A, et al. Inhibition of phosphodiesterase 3, 4, and 5 induces endolymphatic hydrops in mouse inner ear, as evaluated with repeated 9.4T MRI. Acta Otolaryngol,2017,137(1): 8–15. DOI: 10.1080/00016489.2016.1211320
[14] 刘维, 汪芹, 伍伟景. 内耳钆造影MRI技术的原理与临床应用进展. 中国耳鼻咽喉颅底外科杂志,2019,25(4): 439–443. -
期刊类型引用(4)
1. 崔彦儒,郑艳秋,高伟. 阿魏酸钠治疗梅尼埃病模型小鼠的效果及机制研究. 天津医药. 2024(06): 584-588 .  百度学术
百度学术
2. 赵志光,李晓旭,李萌萌,李晓兰,薛建琛. 三维快速液体衰减反转恢复序列(3D-FLAIR)在突发性耳聋临床诊断中的应用价值分析. 中国CT和MRI杂志. 2024(08): 23-25 .  百度学术
百度学术
3. 赵志光,李晓旭,李萌萌,李晓兰,薛建琛. 三维快速液体衰减反转恢复序列(3D-FLAIR)在突发性耳聋临床诊断中的应用价值分析. 中国CT和MRI杂志. 2024(09): 35-37 .  百度学术
百度学术
4. 徐银伟,高婉婷,孙青. 内耳显影对迟发性膜迷路积水诊断的临床效果研究. 生命科学仪器. 2023(S2): 34 .  百度学术
百度学术
其他类型引用(1)

 首页
首页


 下载:
下载: