Cholesterol 7α-Hydroxylase Gene-204A/C Polymorphism in Normal and Gestational Diabetic Pregnancies
-
摘要:目的 探讨胆固醇7α-羟化酶基因(CYP7A1)-204A/C单核苷酸多态性与妊娠糖尿病(GDM)患者及正常孕妇血脂水平等的关系。方法 应用聚合酶链反应-限制性片段长度多态性(PCR-RFLP)技术检测1037例正常妊娠者和627例GDM患者CYP7A1-204A/C基因多态性。酶法测定总胆固醇(TC)、甘油三酯(TG)、高密度脂蛋白胆固醇(HDL-C)、低密度脂蛋白胆固醇(LDL-C)和血糖(Glu),化学发光法测定血浆胰岛素(Ins)。免疫透射比浊法测定载脂蛋白A1(apoA1)和B(apoB)水平。结果 CYP7A1-204A/C多态位点等位基因A、C频率在GDM组和对照组分别为0.586、0.414和0.557、0.443。两组人群基因型频率分布均符合Hardy-Weinberg平衡定律。CYP7A1-204A/C多态性基因型频率、等位基因A、C频率在GDM组和正常对照组间比较差异无统计学意义。正常妊娠对照组CC基因型者较AA型者血浆apoA1水平增高,Ins和HOMA-IR水平降低(P均<0.05);正常妊娠对照组中非肥胖亚组CC基因型者血浆TG水平较AA基因型者增加(P<0.05)。在GDM组CYP7A1基因-204A/C多态性AA基因型者较CC型者孕期增重增加(P<0.05)。结论 CYP7A1基因-204A/C多态性与GDM无关联,但GDM患者CYP7A1基因-204A/C多态性与孕期增重密切相关。该基因位点的变异在正常妊娠孕妇中与血浆apoA1、胰岛素和HOMA-IR水平密切相关,在非肥胖正常妊娠人群中与血浆TG水平增高密切相关。Abstract:Objective To investigate the cholesterol 7α-hydroxylase gene (CYP7A1)-204A/C single nucleotide polymorphism and its relationship with the blood lipid levels of pregnant women with gestational diabetes mellitus (GDM) and normal pregnant women.Methods The genotype and allele frequencies of CYP7A1-204A/C gene polymorphism of 1037 normal pregnant women, the normal controls, and 627 pregnant women with GDM were examined by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis. Total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and blood glucose (Glu) were measured by enzymatic assay. Chemiluminescence determination of plasma insulin (Ins) was conducted. Apolipoproteins A1 (apoA1) and B (apoB) were measured by the turbidimetric immunoassay.Results Allele frequencies of A and C at the CYP7A1-204A/C polymorphic locus were 0.586 and 0.414, respectively, in the GDM group and 0.557 and 0.443, respectively in the control group. The distribution of genotype frequencies in both groups showed conformity with the Hardy-Weinberg principle. There was no significant difference in allele and genotype frequencies between the GDM group and the control group. In the control group, carriers of the genotype AA were associated with significantly higher concentrations of apoA1 and lower levels of Ins and homeostatic model assessment of insulin resistance (HOMA-IR) compared with those with genotype CC (all P<0.05). In the non-obese subgroup of the control subjects, carriers of the genotype CC were associated with significantly higher plasma TG or apoA1 levels compared with those with genotype AA (P<0.05). In the GDM group, carriers with genotype AA of CYP7A1-204A/C polymorphism had elevated levels of gestational weight gain (GWG) compared with those with genotype CC (P<0.05).Conclusion These results suggest that 204A/C polymorphism in the CYP7A1 gene is not associated with GDM, but may be closely associated with gestational weight gain in pregnant women with GDM. Variants in this locus are strongly associated with plasma apoA1, Ins, and HOMA-IR levels in the controls and elevated plasma TG levels in non-obese controls.
-
妊娠糖尿病(gestational diabetes mellitus, GDM)是妊娠期最常见的代谢紊乱性疾病[1],其发病率在我国妊娠妇女人群中达到11.9%~14.8%[2]。GDM的病因目前尚不十分清楚,研究表明该病与遗传因素[3]、血脂异常[4]、氧化应激[5]、炎症[6]等相关。
胆固醇7α-羟化酶(cholesterol 7α-hydroxylase, CYP7A1)是细胞色素P450家族成员之一,该酶是肝脏经典胆汁酸合成途径的限速酶,具有合成并促使胆固醇转换成胆汁酸的功能[7-8]。研究发现CYP7A1基因的缺失变异(del TT 1302-1303)会出现总CYP7A1活性的缺失,导致高胆固醇血症的发生。另外一些研究发现CYP7A1单核苷酸多态性与脂代谢存在关联,并与多种疾病如动脉粥样硬化/冠心病、胆结石以及肠道肿瘤等的发生有关[9]。鉴于血脂水平的变化与CYP7A1基因启动子区-204A/C的单核苷酸多态性密切相关[10],该基因也成为GDM的候选基因。迄今关于CYP7A1基因-204A/C多态性是否与GDM以及妊娠妇女血脂水平等的变化存在关联尚未见报道。本研究就GDM患者及正常孕妇CYP7A1基因-204A/C多态性与临床及相关代谢指标进行分析,对探讨GDM发生的遗传学病因以及妊娠妇女代谢异常的机制具有重要意义。
1. 对象与方法
1.1 对象
GDM组:按国际糖尿病研究协会(IADPSG)推荐的GDM诊断标准确诊的GDM孕妇[11],即孕妇空腹血糖≥5.1 mmol/L,或者餐后1 h≥10.0 mmol/L,或者餐后2 h 血糖≥8.5 mmol/L。对照组:正常妊娠孕妇。以上两组均经询问病史和体检,排除孕前糖尿病及其他妊娠期疾病和多胎妊娠,以及心、肺、肝、肾及其他内分泌疾病,均为来自成都地区的汉族人。该研究经四川大学华西第二医院伦理委员会批准(批准号2017-033),所有研究对象均签署了知情同意书。最终纳入1037例正常妊娠者和627例GDM患者。
1.2 血液基因组DNA的分离及聚合酶链反应扩增
参照常规微量DNA全血提取法从500 μL外周血中分离基因组DNA[12]。聚合酶链反应(polymerase chain reaction, PCR)引物参照文献-合成[13]。引物序列上游5′-AATGTTTTTCCCAGTTCTCTTTC-3′,下游5′-AATTAGCCATTTGTTCATTCT ATTAG-3′,由上海生工生物有限公司合成。PCR反应体系总体积为25 μL,含0.25 μmol引物,12.5 μL Taq PCR 预混液,10 μL双蒸水,0.1 μg DNA模板。PCR反应条件为94 ℃预变性5 min后,94 ℃ 1 min,53 ℃ 30 s,72 ℃ 30 s,28个循环后72 ℃最后延伸7 min。
1.3 PCR扩增产物的消化及电泳
取PCR产物(393 bp)1 μL,加入BsaⅠ限制性内切酶4 U(NEB产品),10倍酶切缓冲液1 μL,加灭菌超纯水配成10 μL体积,于37 ℃消化1 h,取消化产物加入2.5%琼脂糖凝胶板(含Genecolour荧光试剂),在TBE缓冲液中电泳40 min,紫外光下拍照。
1.4 血生化指标分析
使用化学发光法测定血浆Ins,采用酶法试剂盒测定Glu、HDL-C、TG、LDL-C和TC。载脂蛋白A1(apoA1)和载脂蛋白B(apoB)的测定用免疫透射比浊法(ITA)。
胰岛素抵抗水平的分析指标HOMA稳态模型(HOMA-IR)计算方法如下:HOMA-IR=空腹血糖(mmol/L)×空腹胰岛素水平(μU/mL)/22.5 [14]。
1.5 统计学方法
采用SPSS 26.0软件统计,在软件中建立数据库。GDM组和对照组基因型频率采用基因计数法,两组等位基因频率的比较用χ2检验。组间临床指标和代谢水平比较用t检验,不同基因型亚组间的差异用ANOVA分析。CYP7A1基因的基因型进行Hardy-Weinberg 平衡检验。P<0.05为差异有统计学意义。
2. 结果
2.1 GDM组和对照组临床和代谢指标的比较
由表1可见,与正常妊娠对照组比较,GDM组孕妇的孕期增重、TC、LDL-C和apoA1均降低,而在孕周、孕前BMI、空腹血糖、空腹胰岛素、HOMA-IR、TG均升高(P<0.05),年龄、分娩BMI、SBP、DBP、HDL-C和apoB水平两组之间差异无统计学意义。
表 1 GDM组和对照组临床和代谢指标的比较Table 1. Comparison of clinical and metabolic parameters between the GDM and the control groupsIndicator GDM group
(n=627)Control group
(n=1037)P Age/yr. 35.44±3.94 35.74±4.36 0.131 Gestational age/weeks 39.99±0.96 39.22±0.96 0.000 Prepregnancy BMI/(kg/m2) 22.25±3.12 21.17±2.72 0.000 Gestational weight gain/kg 11.50±4.20 13.92±4.25 0.000 Delivery BMI/(kg/m2) 25.81±3.38 26.64±2.70 0.272 SBP/mmHg 115.96±11.04 115.28±10.09 0.189 DBP/mmHg 72.81±8.70 72.23±7.66 0.163 Fasting Ins/(pmol/L) 95.74±118.78 71.32±31.68 0.001 Fasting Glu/(mmol/L) 4.61±0.79 4.39±0.71 0.000 HOMA-IR 3.53±8.91 2.19±2.40 0.000 Triglycerides/(mmol/L) 3.88±1.63 3.66±1.43 0.006 TC/(mmol/L) 5.97±1.24 6.09±1.07 0.047 HDL-C/(mmol/L) 1.98±0.44 2.00±0.41 0.470 LDL-C/(mmol/L) 2.95±0.95 3.20±0.99 0.000 Apo A1/(g/L) 2.29±0.36 2.37±0.43 0.000 Apo B/(g/L) 1.15±0.25 1.15±0.26 0.912 BMI: body mass index; SBP: systolic blood pressere; DBP: diastolic blood pressure; Ins: Insulin; Glu: glucose; HOMA-IR: homeostatic model assessment of insulin resistance; TC: total cholesterol; HDL-C: high-density lipoprotein; LDL-C: low-density lipoprotein; Apo A1: apolipoprotein A1; Apo B1: apolipoprotein B1. 2.2 CYP7A1基因-204A/C多态性分析
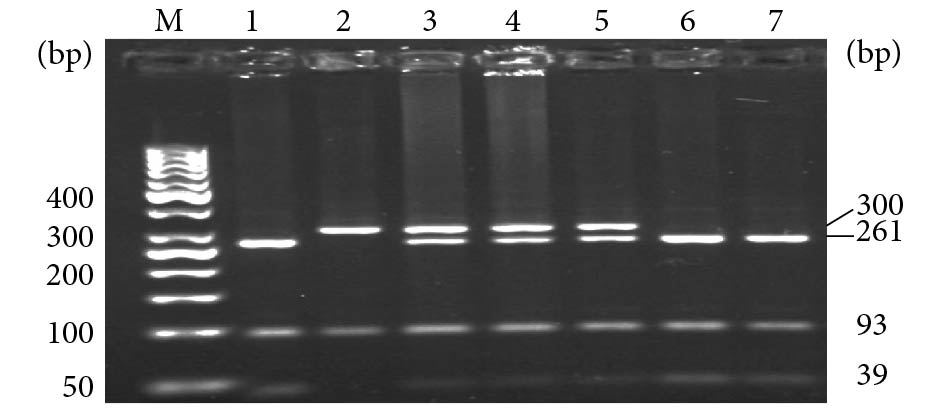
PCR产物经BsaⅠ限制性内切酶水解后,出现3种基因型:AA纯合子显示300 bp和93 bp 2条带;CC纯合子显示261 bp、93 bp和39 bp 3条带;AC杂合子为300 bp,261 bp,93 bp和39 bp 4条带(图1)。
2.3 CYP7A1基因型和等位基因频率的分布和比较
GDM组和对照组CYP7A1-204A/C多态性基因型频率分布均符合Hardy-Weinberg平衡遗传定律(P均>0.05),该位点达到遗传平衡,具有群体代表性。由表2可见,GDM组和对照组A和C等位基因频率分别为0.586、0.414和0.557、0.443。未见两组之间基因型和等位基因频率的分布差异存在统计学意义。
表 2 GDM组和对照组CYP7A1-204A/C基因型及等位基因频率分布Table 2. Distribution of CYP7A1-204A/C genotype and allele frequency in GDM and control groupsItem GDM group/frequency (case), n=627 Control group/frequency (case), n=1037 P Genotype 0.262 AA 0.346 (217) 0.310 (322) AC 0.480 (301) 0.494 (512) CC 0.174 (109) 0.196 (203) Allele 0.112 A 0.586 (735) 0.557 (1156) C 0.414 (519) 0.443 (918) The numbers in the parentheses indicate the number of subjects for each genotype or the number of alleles of each type. 2.4 CYP7A1基因不同基因型亚组间临床和代谢指标的比较
由表3可见,GDM组CYP7A1基因-204位点携带CC基因型者其孕期体质量增加水平低于AA型者(P<0.05)。在GDM组,该位点不同基因型亚组之间代谢指标差异无统计学意义。
表 3 GDM组和对照组中不同CYP7A1-204A/C多态性基因型亚组临床和代谢指标比较Table 3. Comparison of clinical and metabolic parameters of different genotypes of CYP7A1-204A/C polymorphism in GDM and control groupsIndicator GDM group Control group AA (n=217) AC (n=301) CC (n=109) AA (n=322) AC (n=512) CC (n=203) Delivery BMI/(kg/m2) 26.98±3.01 26.76±3.41 26.63±3.95 26.68±2.82 26.73±2.63 26.38±2.66 Gestational weight gain/kg 11.96±4.37 11.44±4.19 10.80±3.84* 13.86±3.63 13.91±4.80 14.03±3.59 Fasting Ins/(pmol/L) 15.32±17.80 15.61±24.98 12.67±8.76 11.50±9.48 10.76±6.98 9.64±4.64** Fasting Glu/(mmol/L) 4.55±0.59 4.63±0.90 4.67±0.77 4.42±0.74 4.38±0.78 4.34±0.39 HOMA-IR 3.32±4.76 3.93±12.06 2.80±2.37 2.46±3.54 2.14±1.84 1.88±0.96* Triglycerides/(mmol/L) 4.00±1.89 3.81±1.50 3.81±1.44 3.66±1.56 3.67±1.35 3.61±1.41 TC/(mmol/L) 5.93±1.14 6.04±1.38 5.89±1.00 6.05±1.18 6.07±1.02 6.19±1.03 HDL-C/(mmol/L) 1.97±0.43 1.99±0.45 1.99±0.42 1.96±0.39 2.02±0.42* 2.00±0.39 LDL-C/(mmol/L) 2.91±0.89 3.02±1.02 2.86±0.83 3.22±1.17 3.14±0.88 3.29±0.90 Apo A1/(g/L) 2.27±0.35 2.31±0.39 2.28±0.33 2.31±0.39 2.39±0.41 2.40±0.51* Apo B/(g/L) 1.14±0.26 1.15±0.24 1.14±0.24 1.16±0.28 1.14±0.24 1.17±0.24 The abbreviations are explained in the note to Table 1. * P<0.05, ** P<0.01, vs. the same group of AA genotype carriers. 在正常妊娠对照组,CYP7A1基因CC基因型携带者其空腹胰岛素(空腹Ins)、HOMA-IR水平较AA型者降低(P<0.05),而apoA1水平较AA型者升高(P<0.05),HDL-C水平在AC型者高于AA型者(P<0.05)(表3)。进一步划分肥胖(BMI≥25 kg/m2)和非肥胖(BMI<25 kg/m2)亚组后(表4),CYP7A1多态性与空腹Ins水平的关系仅在肥胖亚组观察到( P<0.05),而apoA1水平变化的关系仅在非肥胖孕妇观察到(AA型者 vs. CC型者,P<0.05,AA型者vs. AC型者, P<0.01);此外,在非肥胖对照孕妇,CC基因型携带者其血浆TG水平高于AA型携带者(P<0.05)。
表 4 肥胖和非肥胖对照孕妇CYP7A1基因-204A/C多态性不同基因型亚组临床和代谢指标Table 4. Clinical and metabolic parameters of CYP7A1 gene-204A/C genotypes in obese and non-obese control subjectsIndicator Obese control group Non-obese control group AA (n=241) AC (n=376) CC (n=137) AA (n=76) AC (n=129) CC (n=65) Delivery BMI/(kg/m2) 27.76±2.33 27.80±2.11 27.78±1.96 23.37±1.10 23.64±1.05 23.44±1.09 Gestational weight gain/kg 14.38±3.68 14.44±5.15 14.83±3.58 12.23±3.04 12.22±3.07 12.39±3.06 Fasting Ins/(pmol/L) 11.42±7.04 11.00±4.84 10.11±4.82* 11.76±14.48 10.01±11.11 8.74±4.15 Fasting Glu/(mmol/L) 4.41±0.72 4.37±0.42 4.37±0.43 4.50±0.80 4.42±1.36 4.26±0.29 HOMA-IR 2.36±2.72 2.16±1.05 2.00±1.01 2.76±5.27 2.05±3.19 1.66±0.80 Triglycerides/(mmol/L) 3.81±1.70 3.76±1.41 3.56±1.35 3.20±0.92 3.42±1.17 3.73±1.53* TC/(mmol/L) 6.00±1.19 6.01±1.03 6.10±1.03 6.24±1.17 6.24±1.00 6.37±1.00 HDL-C/(mmol/L) 1.93±0.39 1.99±0.40 1.96±0.37 2.02±0.39 2.13±0.44 2.07±0.42 LDL-C/(mmol/L) 3.15±1.20 3.08±0.88 3.28±0.93 3.45±1.09 3.30±0.87 3.33±0.83 Apo A1/(g/L) 2.33±0.39 2.38±0.41 2.38±0.54 2.25±0.39 2.42±0.43** 2.43±0.44* Apo B/(g/L) 1.15±0.27 1.13±0.25 1.15±0.25 1.20±0.31 1.17±0.23 1.19±0.23 The abbreviations are explained in the note to Table 1. * P<0.05, ** P<0.01, vs. the same group of AA genotype carriers. 3. 讨论
本研究首次对CYP7A1基因-204A/C多态性与GDM患者及正常妊娠妇女的关系进行了研究。本研究对成都地区1664例孕妇CYP7A1-204A/C多态性等位基因频率进行了分析,结果显示,C等位基因频率在GDM组和正常妊娠对照组分别为0.414和0.443,与荷兰白人的0.39[10]和美国白人的0.42[15]相近,提示汉族人CYP7A1基因-204A/C多态性位点等位基因频率与欧美白种人没有明显的种族差异。
关于CYP7A1基因-204A/C多态性与血脂水平变化的关系研究较多。本研究在非肥胖对照妊娠妇女发现,CC基因型携带者较AA型者血浆TG水平增加,而在肥胖妊娠妇女则未观察到这种关系,提示该基因多态性在正常妊娠人群中与TG代谢的关系存在不同BMI水平(即肥胖与否)亚组人群之间的差异。关于血清TG代谢受到CYP7A1-204A/C多态性调节的确切机制还未充分确定。在人的观察和动物实验均提示-204A/C多态性通过影响CYP7A1的转录活性而影响胆汁酸合成,后者的增加会使TG的水平增高。例如HOFMAN等[10]在高脂血症和正常人的研究结果显示,使用促进胆汁酸合成的药物消胆胺后,可导致研究对象出现极低密度脂蛋白(VLDL)-TG和VLDL-TC水平增加;小鼠CYP7A1的活性增加可由于缺乏胆固醇-27-羟化酶所致,这些小鼠同时出现肝脏和血清TG水平的增加[16];而在CYP7A1基因敲除小鼠模型上观察到,敲除动物的VLDL合成减少,胆汁酸生物合成出现进一步减少和TG水平的降低[17] CYP7A1-204A/C多态性位点为功能性位点,与A等位基因的启动子转录活性比较,C等位基因的启动子转录活性更高,这可能与C等位基因存在1个Zic3结合位点而增强了CYP7A1的转录活性有关[18]。这些结果说明,CYP7A1的表达及其活性的增加可能导致胆汁酸的合成增多,引起TG水平的升高。本研究结果提示非肥胖对照组孕妇CC基因型携带者与血浆TG水平升高相关,可能在该亚组人群与增加了CC基因型携带者胆汁酸的合成有关。
本研究还发现,对照组及其非肥胖亚组中CC基因型或C等位基因携带者apoA1水平高于AA型者。与WANG等[15]报道的正常人群男性CC基因型个体的HDL-C水平增高相一致,因为apoA1是HDL-C颗粒的主要构成载脂蛋白,与HDL-C的功能密切相关。
本研究在GDM患者组未观察到CYP7A1基因-204A/C多态性与血脂水平的变化存在关联,可能与GDM患者具有显著的胰岛素抵抗而存在更大的脂代谢谱的变化有关,这些变化可能掩盖了上述基因多态性与血脂水平存在关联的效应。
此外,本研究在对照孕妇观察到-204A/C变异与空腹胰岛素和HOMA-IR水平存在关联。LI等[19]研究发现,一些与脂代谢相关基因的变异不仅影响血脂水平,也与葡萄糖代谢的表型(如空腹血糖、糖化血红蛋白和HOMA-IR水平)有关,而基因型对两者的效应呈相反的关系,如TG升高或降低的等位基因分别与后者指标水平的降低或升高有关。本研究在正常非肥胖孕妇的结果显示CC基因型携带者较AA型者TG水平升高,而在正常孕妇CC基因型者较AA型者HOMA-IR水平则降低,与在正常人群观察到的CYP7A1另一SNP位点rs1030431的结果(TG与HOMA-IR水平的变化关系)相似。本研究从该基因的另一功能性SNP位点(rs3808607)方面提供了新的数据。基因变异与糖脂水平多效性关系的机制尚不清楚,涉及到糖脂代谢之间存在的复杂的遗传调控和代谢方面的相互作用关系,需要进一步通过系统生物学的途径加以研究。
值得指出,本研究在GDM孕妇观察到CYP7A1基因-204A/C多态性AA基因型携带者孕期体质量的增加水平较CC基因型者显著增加。在正常对照孕妇未观察到这种关系。在GDM患者存在上述关系的机制尚不清楚。孕期增重(GWG)是一个复杂的表型,受到母体对妊娠应答的影响,例如孕期脂肪的沉集和体积的扩大,以及胎儿的生长、胎盘体积和羊水的产生量等[20-21]。这些单一因素都可能受到母体和胎儿基因以及环境因素方面暴露的影响。近年一项大样本欧洲人群广泛基因组扫描(GWAS)的研究结果显示[22],一些与GWG有关的基因变异与母体有关,其中一些基因变异与母体BMI、血糖和2型糖尿病相关基因的变异相同。本研究发现CYP7A1-204A/C变异与GDM孕期增重的关系,可能是中国汉族人GDM妇女GWG的遗传因素之一,该变异与GDM患者GWG发生关系的进一步研究,有助于为建立遗传因素对妊娠妇女及其子代健康效应的潜在作用关系提供依据。
综上所述,本研究结果表明CYP7A1基因-204A/C变异不是GDM发生的遗传危险因素,但该变异可能与GDM患者孕期增重有关。该基因位点的变异在正常妊娠孕妇中与血浆apoA1、胰岛素和HOMA-IR水平密切相关,在非肥胖正常孕妇人群中与血浆TG水平增高密切相关。
* * *
利益冲突 所有作者均声明不存在利益冲突
-
表 1 GDM组和对照组临床和代谢指标的比较
Table 1 Comparison of clinical and metabolic parameters between the GDM and the control groups
Indicator GDM group
(n=627)Control group
(n=1037)P Age/yr. 35.44±3.94 35.74±4.36 0.131 Gestational age/weeks 39.99±0.96 39.22±0.96 0.000 Prepregnancy BMI/(kg/m2) 22.25±3.12 21.17±2.72 0.000 Gestational weight gain/kg 11.50±4.20 13.92±4.25 0.000 Delivery BMI/(kg/m2) 25.81±3.38 26.64±2.70 0.272 SBP/mmHg 115.96±11.04 115.28±10.09 0.189 DBP/mmHg 72.81±8.70 72.23±7.66 0.163 Fasting Ins/(pmol/L) 95.74±118.78 71.32±31.68 0.001 Fasting Glu/(mmol/L) 4.61±0.79 4.39±0.71 0.000 HOMA-IR 3.53±8.91 2.19±2.40 0.000 Triglycerides/(mmol/L) 3.88±1.63 3.66±1.43 0.006 TC/(mmol/L) 5.97±1.24 6.09±1.07 0.047 HDL-C/(mmol/L) 1.98±0.44 2.00±0.41 0.470 LDL-C/(mmol/L) 2.95±0.95 3.20±0.99 0.000 Apo A1/(g/L) 2.29±0.36 2.37±0.43 0.000 Apo B/(g/L) 1.15±0.25 1.15±0.26 0.912 BMI: body mass index; SBP: systolic blood pressere; DBP: diastolic blood pressure; Ins: Insulin; Glu: glucose; HOMA-IR: homeostatic model assessment of insulin resistance; TC: total cholesterol; HDL-C: high-density lipoprotein; LDL-C: low-density lipoprotein; Apo A1: apolipoprotein A1; Apo B1: apolipoprotein B1. 表 2 GDM组和对照组CYP7A1-204A/C基因型及等位基因频率分布
Table 2 Distribution of CYP7A1-204A/C genotype and allele frequency in GDM and control groups
Item GDM group/frequency (case), n=627 Control group/frequency (case), n=1037 P Genotype 0.262 AA 0.346 (217) 0.310 (322) AC 0.480 (301) 0.494 (512) CC 0.174 (109) 0.196 (203) Allele 0.112 A 0.586 (735) 0.557 (1156) C 0.414 (519) 0.443 (918) The numbers in the parentheses indicate the number of subjects for each genotype or the number of alleles of each type. 表 3 GDM组和对照组中不同CYP7A1-204A/C多态性基因型亚组临床和代谢指标比较
Table 3 Comparison of clinical and metabolic parameters of different genotypes of CYP7A1-204A/C polymorphism in GDM and control groups
Indicator GDM group Control group AA (n=217) AC (n=301) CC (n=109) AA (n=322) AC (n=512) CC (n=203) Delivery BMI/(kg/m2) 26.98±3.01 26.76±3.41 26.63±3.95 26.68±2.82 26.73±2.63 26.38±2.66 Gestational weight gain/kg 11.96±4.37 11.44±4.19 10.80±3.84* 13.86±3.63 13.91±4.80 14.03±3.59 Fasting Ins/(pmol/L) 15.32±17.80 15.61±24.98 12.67±8.76 11.50±9.48 10.76±6.98 9.64±4.64** Fasting Glu/(mmol/L) 4.55±0.59 4.63±0.90 4.67±0.77 4.42±0.74 4.38±0.78 4.34±0.39 HOMA-IR 3.32±4.76 3.93±12.06 2.80±2.37 2.46±3.54 2.14±1.84 1.88±0.96* Triglycerides/(mmol/L) 4.00±1.89 3.81±1.50 3.81±1.44 3.66±1.56 3.67±1.35 3.61±1.41 TC/(mmol/L) 5.93±1.14 6.04±1.38 5.89±1.00 6.05±1.18 6.07±1.02 6.19±1.03 HDL-C/(mmol/L) 1.97±0.43 1.99±0.45 1.99±0.42 1.96±0.39 2.02±0.42* 2.00±0.39 LDL-C/(mmol/L) 2.91±0.89 3.02±1.02 2.86±0.83 3.22±1.17 3.14±0.88 3.29±0.90 Apo A1/(g/L) 2.27±0.35 2.31±0.39 2.28±0.33 2.31±0.39 2.39±0.41 2.40±0.51* Apo B/(g/L) 1.14±0.26 1.15±0.24 1.14±0.24 1.16±0.28 1.14±0.24 1.17±0.24 The abbreviations are explained in the note to Table 1. * P<0.05, ** P<0.01, vs. the same group of AA genotype carriers. 表 4 肥胖和非肥胖对照孕妇CYP7A1基因-204A/C多态性不同基因型亚组临床和代谢指标
Table 4 Clinical and metabolic parameters of CYP7A1 gene-204A/C genotypes in obese and non-obese control subjects
Indicator Obese control group Non-obese control group AA (n=241) AC (n=376) CC (n=137) AA (n=76) AC (n=129) CC (n=65) Delivery BMI/(kg/m2) 27.76±2.33 27.80±2.11 27.78±1.96 23.37±1.10 23.64±1.05 23.44±1.09 Gestational weight gain/kg 14.38±3.68 14.44±5.15 14.83±3.58 12.23±3.04 12.22±3.07 12.39±3.06 Fasting Ins/(pmol/L) 11.42±7.04 11.00±4.84 10.11±4.82* 11.76±14.48 10.01±11.11 8.74±4.15 Fasting Glu/(mmol/L) 4.41±0.72 4.37±0.42 4.37±0.43 4.50±0.80 4.42±1.36 4.26±0.29 HOMA-IR 2.36±2.72 2.16±1.05 2.00±1.01 2.76±5.27 2.05±3.19 1.66±0.80 Triglycerides/(mmol/L) 3.81±1.70 3.76±1.41 3.56±1.35 3.20±0.92 3.42±1.17 3.73±1.53* TC/(mmol/L) 6.00±1.19 6.01±1.03 6.10±1.03 6.24±1.17 6.24±1.00 6.37±1.00 HDL-C/(mmol/L) 1.93±0.39 1.99±0.40 1.96±0.37 2.02±0.39 2.13±0.44 2.07±0.42 LDL-C/(mmol/L) 3.15±1.20 3.08±0.88 3.28±0.93 3.45±1.09 3.30±0.87 3.33±0.83 Apo A1/(g/L) 2.33±0.39 2.38±0.41 2.38±0.54 2.25±0.39 2.42±0.43** 2.43±0.44* Apo B/(g/L) 1.15±0.27 1.13±0.25 1.15±0.25 1.20±0.31 1.17±0.23 1.19±0.23 The abbreviations are explained in the note to Table 1. * P<0.05, ** P<0.01, vs. the same group of AA genotype carriers. -
[1] ACOG Practice Bulletin. No. 190: Gestational diabetes mellitus. Obstet Gynecol,2018,131(2): e49–e64. DOI: 10.1097/AOG.0000000000002501
[2] JUAN J, YANG H. Prevalence, prevention, and lifestyle intervention of gestational diabetes mellitus in China. Int J Environ Res Public Health,2020,17(24): 9517. DOI: 10.3390/ijerph17249517
[3] ZHANG C, BAO W, RONG Y, et al. Genetic variants and the risk of gestational diabetes mellitus: a systematic review. Hum Reprod Update,2013,19(4): 376–390. DOI: 10.1093/humupd/dmt013
[4] RYCKMAN K K, SPRACKLEN C N, SMITH C J, et al. Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. BJOG,2015,122(5): 643–651. DOI: 10.1111/1471-0528.13261
[5] LÓPEZ-TINOCO C, ROCA M, GARCÍA-VALERO A, et al. Oxidative stress and antioxidant status in patients with late-onset gestational diabetes mellitus. Acta Diabetol,2013,50(2): 201–208. DOI: 10.1007/s00592-011-0264-2
[6] MRIZAK I, ARFA A, FEKIH M, et al. Inflammation and impaired endothelium-dependant vasodilatation in non obese women with gestational diabetes mellitus: preliminary results. Lipids Health Dis,2013,12: 93. DOI: 10.1186/1476-511X-12-93
[7] RUSSELL D W. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem,2003,72: 137–174. DOI: 10.1146/annurev.biochem.72.121801.161712
[8] RUSSELL D W, SETCHELL K D. Bile acid biosynthesis. Biochemistry,1992,31(20): 4737–4749. DOI: 10.1021/bi00135a001
[9] LORBEK G, LEWINSKA M, ROZMAN D. Cytochrome P450s in the synthesis of cholesterol and bile acids-from mouse models to human diseases. FEBS J,2012,279(9): 1516–1533. DOI: 10.1111/j.1742-4658.2011.08432.x
[10] HOFMAN M K, GROENENDIJK M, VERKUIJLEN P J, et al. Modulating effect of the A-278C promoter polymorphism in the cholesterol 7alpha-hydroxylase gene on serum lipid levels in normolipidaemic and hypertriglyceridaemic individuals. Eur J Hum Genet,2004,12(11): 935–941. DOI: 10.1038/sj.ejhg.5201236
[11] ZHOU M, LIU X H, LIU Q Q, et al. Lactonase activity, status, and genetic variations of paraoxonase 1 in women with gestational diabetes mellitus. J Diabetes Res,2020,2020: 3483427. DOI: 10.1155/2020/3483427
[12] GUAN L, FAN P, LIU X, et al. Maternal GALNT2 variations affect blood pressure, atherogenic index, and fetal growth, depending on BMI in gestational diabetes mellitus. Front Endocrinol (Lausanne),2021,12: 690229. DOI: 10.3389/fendo.2021.690229
[13] YE M, SUN J, CHEN Y, et al. Response of serum LDL cholesterol to oatmeal consumption depends on CYP7A1 rs3808607 genotype in Chinese. Asia Pac J Clin Nutr,2020,29(2): 423–433. DOI: 10.6133/apjcn.202007_29(2).0025
[14] MATTHEWS D R, HOSKER J P, RUDENSKI A S, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia,1985,28(7): 412–419. DOI: 10.1007/BF00280883
[15] WANG J, FREEMAN D J, GRUNDY S M, et al. Linkage between cholesterol 7alpha-hydroxylase and high plasma low-density lipoprotein cholesterol concentrations. J Clin Invest,1998,101(6): 1283–1291. DOI: 10.1172/JCI1343
[16] REPA J J, LUND E G, HORTON J D, et al. Disruption of the sterol 27-hydroxylase gene in mice results in hepatomegaly and hypertriglyceridemia. Reversal by cholic acid feeding. J Biol Chem,2000,275(50): 39685–39692. DOI: 10.1074/jbc.M007653200
[17] POST S M, GROENENDIJK M, Van Der HOOGT C C, et al. Cholesterol 7alpha-hydroxylase deficiency in mice on an APOE*3-Leiden background increases hepatic ABCA1 mRNA expression and HDL-cholesterol. Arterioscler Thromb Vasc Biol,2006,26(12): 2724–2730. DOI: 10.1161/01.ATV.0000247260.42560.e1
[18] 陈玉娟,张思仲,肖翠英,等. CYP7A1基因-204位点A/C变异对启动子活性的影响. 中国生物化学与分子生物学报,2006,22(6): 450–453. DOI: 10.3969/j.issn.1007-7626.2006.06.004 [19] LI N, Van Der SIJDE M R, BAKKER S J, et al. Pleiotropic effects of lipid genes on plasma glucose, HbA1c, and HOMA-IR levels. Diabetes,2014,63(9): 3149–3158. DOI: 10.2337/db13-1800
[20] LAWLOR D A, RELTON C, SATTAR N, et al. Maternal adiposity--a determinant of perinatal and offspring outcomes? Nat Rev Endocrinol,2012,8(11): 679–688. DOI: 10.1038/nrendo.2012.176
[21] LAWLOR D A. The Society for Social Medicine John Pemberton Lecture 2011. Developmental overnutrition--an old hypothesis with new importance? Int J Epidemiol,2013,42(1): 7–29. DOI: 10.1093/ije/dys209
[22] WARRINGTON N M, RICHMOND R, FENSTRA B, et al. Maternal and fetal genetic contribution to gestational weight gain. Int J Obes (Lond),2018,42(4): 775–784. DOI: 10.1038/ijo.2017.248
-
期刊类型引用(8)
1. 林培叶,邓海燕,陈韡. 糖尿病患者在生化免疫检验过程中运用化学发光免疫测定技术检验的临床价值. 糖尿病新世界. 2024(04): 47-49 .  百度学术
百度学术
2. 郎肖玲,何侃,李松阳,曾奕,陈颖姣,朱惠,郭朝容,李德华,杨兰. 基于价值医疗理念提升门诊服务质量——以四川省某三级甲等医院为例. 中国卫生事业管理. 2024(06): 632-635+685 .  百度学术
百度学术
3. 高若瑄,屈梦君,赵红. 微信平台路径健康宣教在妊娠期糖尿病患者中的应用. 国际医药卫生导报. 2024(11): 1928-1932 .  百度学术
百度学术
4. 张凤娇,陈燕华,连小英. 地特胰岛素与精蛋白人胰岛素治疗妊娠期糖尿病的疗效及对患者血糖水平的影响. 糖尿病新世界. 2024(15): 95-97+101 .  百度学术
百度学术
5. 田艳闽,梁翠瑛,江琳. FMEA护理模式联合孕早期营养干预对妊娠糖尿病患者血糖血脂水平的影响. 吉林医学. 2024(11): 2793-2796 .  百度学术
百度学术
6. 冯淼. 化学发光免疫检测在生化检验中的应用. 当代化工研究. 2023(15): 74-76 .  百度学术
百度学术
7. 周卓,穆宝妮,冯思思,段小霞. 胆固醇7α-羟化酶基因与妊娠期增重及妊娠结局相关性研究. 陕西医学杂志. 2023(08): 992-996 .  百度学术
百度学术
8. 唐芳梅,白怀,关林波,刘兴会,范平,周密,吴玉洁,刘思旭,王玉峰,李德华. 妊娠糖尿病载脂蛋白C3基因Sst Ⅰ多态性与血脂关系的研究. 四川大学学报(医学版). 2023(05): 994-999 .  百度学术
百度学术
其他类型引用(0)

 首页
首页


 下载:
下载: