Prognostic Value of the Expression of Myeloid Leukemia Factor 1-Interacting Protein in Gastric Cancer and Its Regulatory Role in Tumor Progression
-
摘要:目的 探讨胃癌组织中骨髓增生异常白血病因子1相互作用蛋白(MLF1IP)表达的预后价值及其在肿瘤进展中的调控作用。方法 采用GEO、Kaplan-Meier Plotter数据库分析胃癌肿瘤组织中MLF1IP的表达水平及其与患者预后的关系;回顾性分析2015年1–12月在我院行胃癌根治术的患者108例,检测胃癌及癌旁组织中MLF1IP的表达情况;分析MLF1IP与胃癌患者临床病理参数间的关系及其对患者远期预后的影响;进一步采用单因素与多因素回归分析胃癌患者远期预后的危险因素;ROC曲线分析MLF1IP对胃癌远期预后的评估价值。选择胃癌细胞系MGC803体外分析MLF1IP对癌细胞增殖、迁移与侵袭能力的影响。建立裸鼠移植瘤模型分析在体环境下MLF1IP对肿瘤生长的影响。结果 GEO数据库胃癌队列GSE29272结果显示胃癌组织中MLF1IP表达水平高于正常组织(P<0.05),Kaplan-Meier Plotter数据库提示MLF1IP高表达患者预后不良。免疫组化分析显示,胃癌组织中MLF1IP表达水平高于癌旁组织(P<0.05);相关性分析显示MLF1IP水平与胃癌组织中Ki67(r=0.609,P<0.01)、外周血癌胚抗原(carcinoembryonic antigen, CEA)(r=0.572,P<0.01)及糖类抗原19-9(carbohydrate antigen 19-9, CA19-9)(r=0.623,P<0.01)均呈正相关;Kaplan-Meier(K-M)生存分析显示MLF1IP高表达组患者术后5年生存率低于MLF1IP低表达组(P<0.01);Cox回归分析显示,MLF1IP表达量(HR=2.508,95%CI:1.259~4.999)、CEA≥5 μg/L(HR=2.171,95%CI:1.152~4.092)、CA19-9≥37 kU/L(HR=2.401,95%CI:1.094~5.269)、T分期为3~4期(HR=2.779,95%CI:1.049~7.358)及N分期为2~3期(HR=2.072,95%CI:1.100~3.904)均是影响胃癌根治术后5年生存率的独立危险因素;ROC分析表明MLF1IP(以蛋白相对表达量3.00为截点值)评估胃癌患者根治术后5年生存率的敏感性为75.00%,特异性为76.92%,准确度为76.2%(P<0.05)。CCK-8、Transwell及划痕实验证明,体外干扰MLF1IP基因表达可显著抑制胃癌细胞的增殖、迁移和侵袭。裸鼠皮下移植瘤实验显示,干扰MLF1IP基因可显著抑制肿瘤的生长。结论 MLF1IP在胃癌组织中表达升高并对患者预后不良有一定的预测价值,其可能参与了胃癌细胞增殖、迁移和侵袭等恶性行为。Abstract:Objective To investigate the prognostic value of the expression of myeloid leukemia factor 1-interacting protein (MLF1IP) in gastric cancer tissue and its regulatory role in tumor progression.Methods Gene Expression Omnibus (GEO) database was used to analyze the expression level of MLF1IP in tumor tissues of gastric cancer patients. Kaplan-Meier Plotter database was used to analyze the relationship between MLF1IP expression level and patient prognosis. We conducted a retrospective analysis of 108 gastric cancer patients who had undergone radical surgery at our hospital between January 2015 and December 2015. The expression of MLF1IP in gastric cancer tissue and adjacent tissues was examined. We analyzed the relationship between MLF1IP and the clinicopathological parameters of gastric cancer patients and its impact on the long-term prognosis of gastric cancer patients. Univariate and multivariate regression analyses were done to identify the risk factors affecting the long-term prognosis of gastric cancer patients. The assessment value of MLF1IP for long-term prognosis of gastric cancer was analyzed with ROC curve. The effects of MLF1IP on the proliferation, migration, and invasion of gastric cancer cells were analyzed in vitro with gastric cancer cell line (MGC803). A xenograft tumor model was established with nude mice to analyze in vivo the effect of MLF1IP on tumor growth.Results The results of the gastric cancer cohort GSE29272 of GEO database showed that the expression level of MLF1IP in gastric cancer tissues was significantly higher than that in normal tissues (P<0.05). Analysis with Kaplan-Meier Plotter database indicated that high MLF1IP expression was correlated with poor prognosis in gastric cancer patients. Immunohistochemical analysis showed that the expression level of MLF1IP in gastric cancer tissues was higher than that in adjacent tissues (P<0.05). Correlation analysis showed that the MLF1IP level in gastric cancer tissue was positively correlated with Ki67 (r=0.609, P<0.01), peripheral blood carcinoembryonic antigen (CEA) (r=0.572, P<0.01) and carbohydrate antigen 19-9 (CA19-9) (r=0.623, P<0.01). Kaplan-Meier (K-M) survival analysis showed that the 5-year survival rate of patients in the MLF1IP high expression group was significantly lower than that in the MLF1IP low expression group (P<0.01). Cox regression analysis showed that independent risk factors for 5-year survival after radical gastrectomy for gastric cancer included the expression of MLF1IP (HR=2.508, 95% CI: 1.259-4.999), CEA≥5 μg/L (HR=2.171, 95% CI: 1.152-4.092), CA19-9≥37 kU/L (HR=2.401, 95% CI: 1.094-5.269), and T3-T4 stages (HR=2.779, 95% CI: 1.049-7.358) and N2-N3 stages (HR=2.072, 95% CI: 1.100-3.904). ROC analysis showed that the sensitivity, specificity, and accuracy of MLF1IP (the cut-off value was 3.00 relative protein expression level) in assessing the 5-year survival rate after radical gastrectomy for gastric cancer was 75.00%, 76.92%, and 76.2%, respectively (P<0.05). CCK-8, Transwell assay, and scratch assays showed that in vitro knocking down of MLF1IP gene expression significantly inhibited the proliferation, migration and invasion of gastric cancer cells. Subcutaneous tumor xenograft experiment in nude mice showed that knocking down MLF1IP gene significantly inhibited tumor growth.Conclusion Increased expression of MLF1IP in gastric cancer tissue, which may be involved in the malignant activities of proliferation, migration, and invasion of gastric cancer cells, has a certain predictive value for poor prognosis.
-
Keywords:
- Gastric cancer /
- MLF1IP /
- Prognosis /
- Proliferation /
- Migration /
- Invasion
-
胃癌是常见的消化系统恶性肿瘤,其发病率和死亡率均位于我国恶性肿瘤的前列[1]。近年来,尽管胃癌的综合治疗水平得到不断提升,但胃癌患者术后5年生存率并没有获得显著改善,这很大程度上归咎于临床上对患者预后的评估仍存在一定的局限性[2-3]。肿瘤微环境下存在大量的分子表达异常,各种分子间可通过复杂的信号传递参与肿瘤的发生与发展[4]。因此,分子病理学水平的研究有望为探寻评估胃癌患者远期预后的生物学指标提供新的研究思路。骨髓增生异常白血病因子1相互作用蛋白(myeloid leukemia factor 1-interacting protein, MLF1IP)是一种能够与着丝粒结合的功能蛋白,对维持细胞正常的生理功能具有重要作用[5]。新近研究发现,MLF1IP在乳腺癌、非小细胞肺癌等多种恶性肿瘤中高表达[6-7],且与患者预后不良密切相关。但MLF1IP在胃癌中表达情况的研究未见报道,因此,本研究尝试分析MLF1IP在胃癌组织中的表达情况,并进一步研究其对胃癌细胞恶性生物学行为的影响,以期为胃癌患者的预后评估提供潜在的分子标志物。
1. 资料与方法
1.1 临床资料
选取2015年1–12月在蚌埠医学院第一附属医院接受胃癌根治术治疗的患者。纳入标准:①确诊为原发性胃癌;②均成功接受胃癌根治术;③有完整的病例资料;④知情同意;⑤支持后期回访工作。排除标准:①合并其他恶性肿瘤;②患有严重的肝、肾或心血管疾病。最终依据以上标准,纳入患者108例,获取患者如下信息:①基线资料:包括年龄、性别、术前肿瘤标志物表达水平、肿瘤细胞类型、肿瘤大小、临床分期、淋巴结转移等信息;②生存资料:通过门诊复查、电话随访等渠道获取患者术后5年生存信息;③手术蜡块:收集患者癌组织和癌旁组织蜡块用于后续免疫组化染色。本研究获蚌埠医学院伦理委员会的批准(伦科批字[2021]第203号)。
1.2 主要试剂和仪器
人胃癌细胞系MGC803来源于国家生物医学实验细胞资源库(资源编号:1101HUM-PUMC000660);兔源抗MLF1IP多克隆抗体购自武汉三鹰公司;兔源抗Ki67、β-actin单克隆抗体购自美国Abcam公司;HRP标记抗兔二抗购自北京中杉金桥生物技术有限公司;胎牛血清、RPMI1640培养基、抗青霉素链霉素双抗混合液、PBS缓冲液、胰酶(含0.02%EDTA)购自美国GIBCO公司;CCK-8试剂盒购自北京索莱宝科技有限公司;SDS-PAGE凝胶试剂盒购自上海酶联生物科技有限公司;基质胶和Transwell小室购自美国Corning公司;特异性过表达和干扰MLF1IP基因的慢病毒载体及对照空载质粒由上海吉凯基因化学技术有限公司构建。倒置成像显微镜购自德国蔡司公司。
1.3 检测方法
1.3.1 GEO数据库胃癌队列GSE29272分析MLF1IP在胃癌组织及癌旁组织中的表达水平
GSE29272队列包括134组配对的胃癌及正常样本,数据经由RMA方法进行标准化。生存分析由Kaplan-Meier Plotter数据库完成,以MLF1IP(探针:218883_s_at)基因表达量中位数为标准,将胃癌队列划分为MLF1IP高表达组和MLF1IP低表达组,并进行生存分析。
1.3.2 免疫组化分析MLF1IP及Ki67在胃癌组织和癌旁组织中的表达情况
收集108例胃癌患者肿瘤组织和癌旁组织蜡块,切片后进行免疫组织化学检测。简述如下:切片经脱蜡后,依次进行抗原修复、阻断、封闭、4 ℃孵育一抗MLF1IP(1∶100,Proteintech)或Ki67(1∶200,Abcam)、孵育二抗,再经DAB显色、复染细胞核后进行脱水封片。采用Image-Pro Plus6.0软件计算相对积分光密度(IOD)值。
1.3.3 细胞转染技术构建MLF1IP基因高表达和低表达胃癌细胞系
将胃癌细胞系(MGC803)接种于6孔板中,并于37 ℃、体积分数为5%CO2培养箱中培养24 h。待细胞密度达70%~80%时分别用过表达MLF1IP载体和对照空载体、干扰MLF1IP载体(siRNA-ACGTTCAAAGAACACTTTAGAAA)和对照空载体对MGC803细胞进行转染,并使用嘌呤霉素筛选稳定表达的细胞株用于后续实验研究。
1.3.4 Western blot验证MLF1IP基因转染效果
收集转染成功后的MGC803细胞,使用RIPA裂解液裂解细胞并提取蛋白,经SDS-PAG电泳、转膜及封闭后,滴加一抗MLF1IP(1∶2000)或β-actin(内参,1∶1000),并于4 ℃孵育过夜;再经TBST洗膜、二抗孵育、ECL超敏发光液显色及凝胶成像系统采集图片。使用ImageJ软件分析目标条带光密度值与内参条带光密度值的比值,作为目标蛋白的相对表达量。
1.3.5 CCK-8实验评估MLF1IP对胃癌细胞增殖的影响
将转染成功后的MGC803细胞按照2×103/孔的密度接种到96孔板,每孔加入10 μL CCK-8试剂,培养箱孵育3 h,酶标仪检测450 nm处吸光度值。
1.3.6 Transwell实验及划痕实验评估MLF1IP对胃癌细胞迁移能力的影响
①Transwell实验:使用Transwell小室,上室接种转染成功后的MGC803细胞(2.5×105 mL-1,200 μL),下室加入600 μL含20%FBS的DMEM培养基,并于37 ℃、体积分数为5%CO2培养箱中培养24 h;再经体积分数为4%多聚甲醛固定,结晶紫染色,最后于倒置显微镜下计数迁移细胞数量。②划痕实验:将转染成功后的MGC803细胞以2×105/孔均匀地接种至6孔板中,24 h后用移液器枪头在孔中制造划痕,PBS冲洗后,更换完全培养基,分别在培养0 h与24 h后于显微镜下拍照,并计算细胞迁移率。迁移率=〔(迁移前的划痕距离-迁移后的划痕距离)/迁移前的划痕距离〕×100%,每组选择3个视野,计算平均数,实验重复3次以上。
1.3.7 Transwell实验评估MLF1IP对胃癌细胞侵袭能力的影响
取40 μL配制好的基质胶包被Transwell小室上室,待其凝固后接种转染成功的200 μL MGC803细胞(2.5×105 mL-1),下室加入600 μL含20%FBS的DMEM培养基,后续检测同1.3.6,计数侵袭细胞数量。
1.3.8 裸鼠移植瘤模型的构建
选取4~6周龄,以BALB/c为遗传背景的裸鼠,购自江苏集萃药康生物科技股份有限公司,于无特定病原菌(specific pathogen free, SPF)环境下饲养。将9只裸鼠随机分为Control组、si-MLF1IP组及LV-MLF1IP组,每组3只。敲低和过表达MLF1IP基因后的MGC803细胞经0.25%胰蛋白酶消化,离心后,用无血清DMEM培养基将细胞悬液密度调整为5×107 mL-1,取200 μL分别接种于si-MLF1IP组和LV-MLF1IP组裸鼠皮下,而Control组接种未经MLF1IP基因干预的MGC803细胞。日常观察裸鼠状态及肿瘤体积变化(肿瘤体积=1/2×长×宽2)。21 d后用颈椎脱臼法处死裸鼠,解剖分离瘤体,拍照并记录。本实验通过蚌埠医学院动物研究伦理委员会批准(伦动科批字[2021]第283号)。
1.4 统计学方法
计量数据采用
$ \bar x \pm s $ 表示,采用t检验进行两组间比较;采用Spearman检验进行相关性分析;采用χ2检验进行率的比较;采用Kaplan-Meier(K-M)法进行生存分析(组间生存率比较采用Log-rank χ2检验);采用Cox比例风险回归模型分析影响胃癌患者根治术后5年生存率的独立危险因素;采用ROC曲线分析MLF1IP预测胃癌患者术后5年生存率的诊断价值。α双侧=0.05。2. 结果
2.1 MLF1IP基因在胃癌组织中高表达且与患者预后不良相关
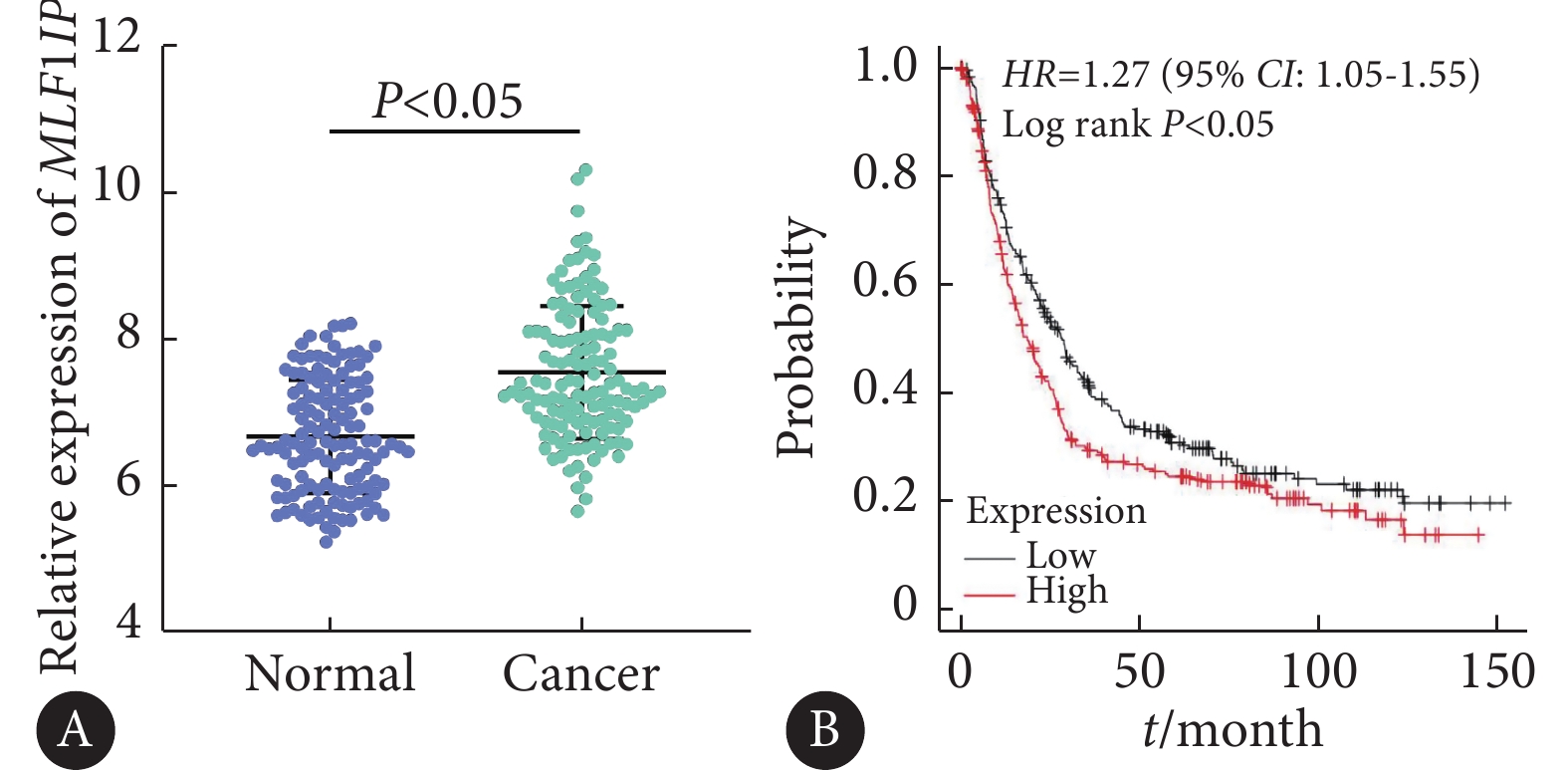
如图1,通过分析GEO数据库胃癌队列GSE29272,发现MLF1IP基因在胃癌组织中的表达水平高于正常组织,差异有统计学意义(P<0.05)。进一步采用Kaplan-Meier Plotter数据库分析发现,MLF1IP基因高表达的胃癌患者预后较低表达患者差。
2.2 MLF1IP在胃癌组织中的表达显著上调
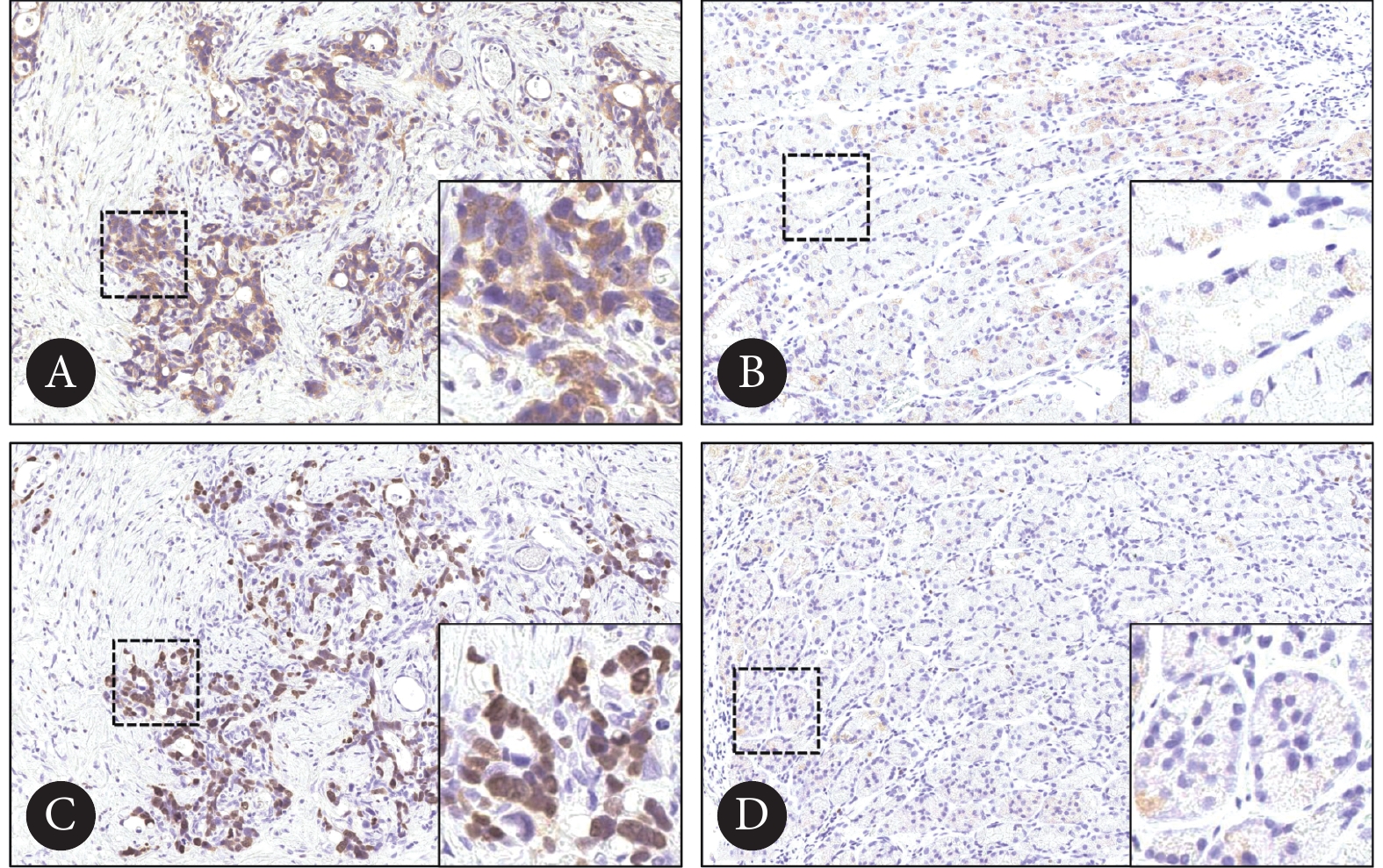
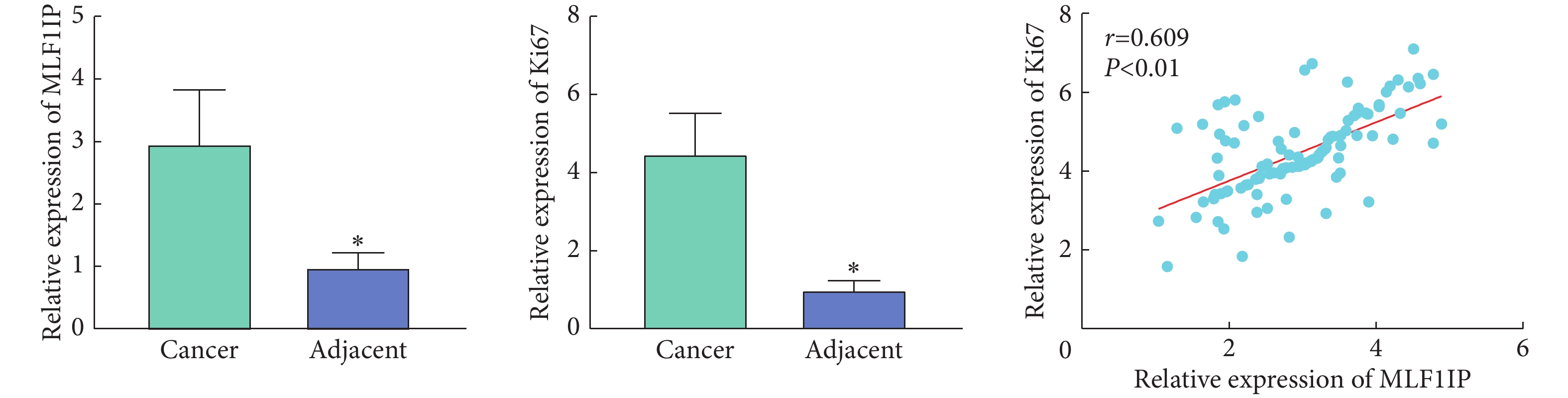
如图2、图3,免疫组化染色结果显示,与癌旁组织对比,MLF1IP及Ki67在胃癌组织中表达量增加(P<0.05);胃癌组织中MLF1IP与Ki67的相对IOD值呈正相关(r=0.609, P<0.01)。
![]() 图 2 胃癌及癌旁组织中MLF1IP及Ki67的表达情况Figure 2. Expression of MLF1IP and Ki67 in gastric cancer tissue and the adjacent tissueA, B: Immunohistochemical staining of MLF1IP in gastric cancer tissue (×100, A) and the adjacent tissue (×400, B); C, D: immunohistochemical staining of Ki67 in gastric cancer tissue (×100, C) and the adjacent tissue (×400, D).
图 2 胃癌及癌旁组织中MLF1IP及Ki67的表达情况Figure 2. Expression of MLF1IP and Ki67 in gastric cancer tissue and the adjacent tissueA, B: Immunohistochemical staining of MLF1IP in gastric cancer tissue (×100, A) and the adjacent tissue (×400, B); C, D: immunohistochemical staining of Ki67 in gastric cancer tissue (×100, C) and the adjacent tissue (×400, D).2.3 MLF1IP在胃癌组织中的表达量与患者临床病理特征的关系
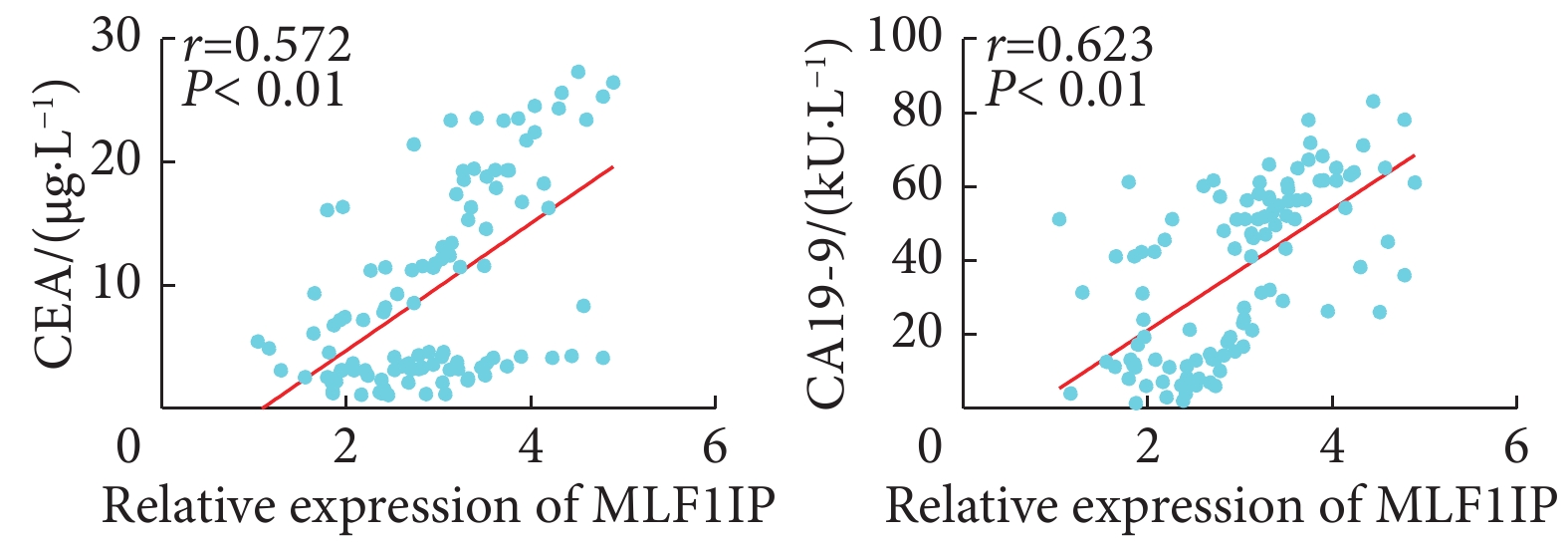
以MLF1IP蛋白在胃癌组织中的相对表达量的中位数(3.00)为界,将108例胃癌患者分为MLF1IP低表达组和MLF1IP高表达组。表1可见,MLF1IP表达量与患者性别、年龄、肿瘤大小及癌细胞组织学类型无关(P>0.05);MLF1IP高表达组患者癌胚抗原(carcinoembryonic antigen, CEA)≥5 μg/L、糖类抗原19-9(carbohydrate antigen 19-9, CA19-9)≥37 kU/L、T分期为3~4期和N分期为2~3期的比例较MLF1IP低表达组增加(P<0.05)。进一步相关性分析发现(图4),MLF1IP在胃癌组织中的表达量与CEA(r=0.572, P<0.01)及CA19-9(r=0.623, P<0.01)的表达量呈正相关。
表 1 108例胃癌患者的基线资料Table 1. The baseline data of 108 patients with gastric cancerCharacteristic n MLF1IP expression P Low (n=54) High (n=54) Sex/case (%) 0.837 Male 73 37 (50.7) 36 (49.3) Female 35 17 (48.6) 18 (51.4) Age/case (%) 0.077 <60 yr. 65 28 (43.1) 37 (56.9) ≥60 yr. 43 26 (60.5) 17 (39.5) CEA/case (%) <0.01 <5 μg/L 52 34 (65.4) 18 (34.6) ≥5 μg/L 56 20 (35.7) 36 (64.3) CA19-9/case (%) <0.01 <37 kU/L 51 40 (78.4) 11 (21.6) ≥37 kU/L 57 14 (24.6) 43 (75.4) Tumor size/case (%) 0.053 <5 cm 60 35 (58.3) 25 (41.7) ≥5 cm 48 19 (39.6) 29 (60.4) Histological type/case (%) 0.139 Adenocarcinoma 95 45 (47.4) 50 (52.6) Other 13 9 (69.2) 4 (30.8) T stage/case (%) <0.01 1-2 37 28 (75.7) 9 (24.3) 3-4 71 26 (36.6) 45 (63.4) N stage/case (%) <0.01 0-1 59 37 (62.7) 22 (37.3) 2-3 49 17 (34.7) 32 (65.3) 2.4 胃癌组织中MLF1IP高表达的患者术后5年生存率显著降低
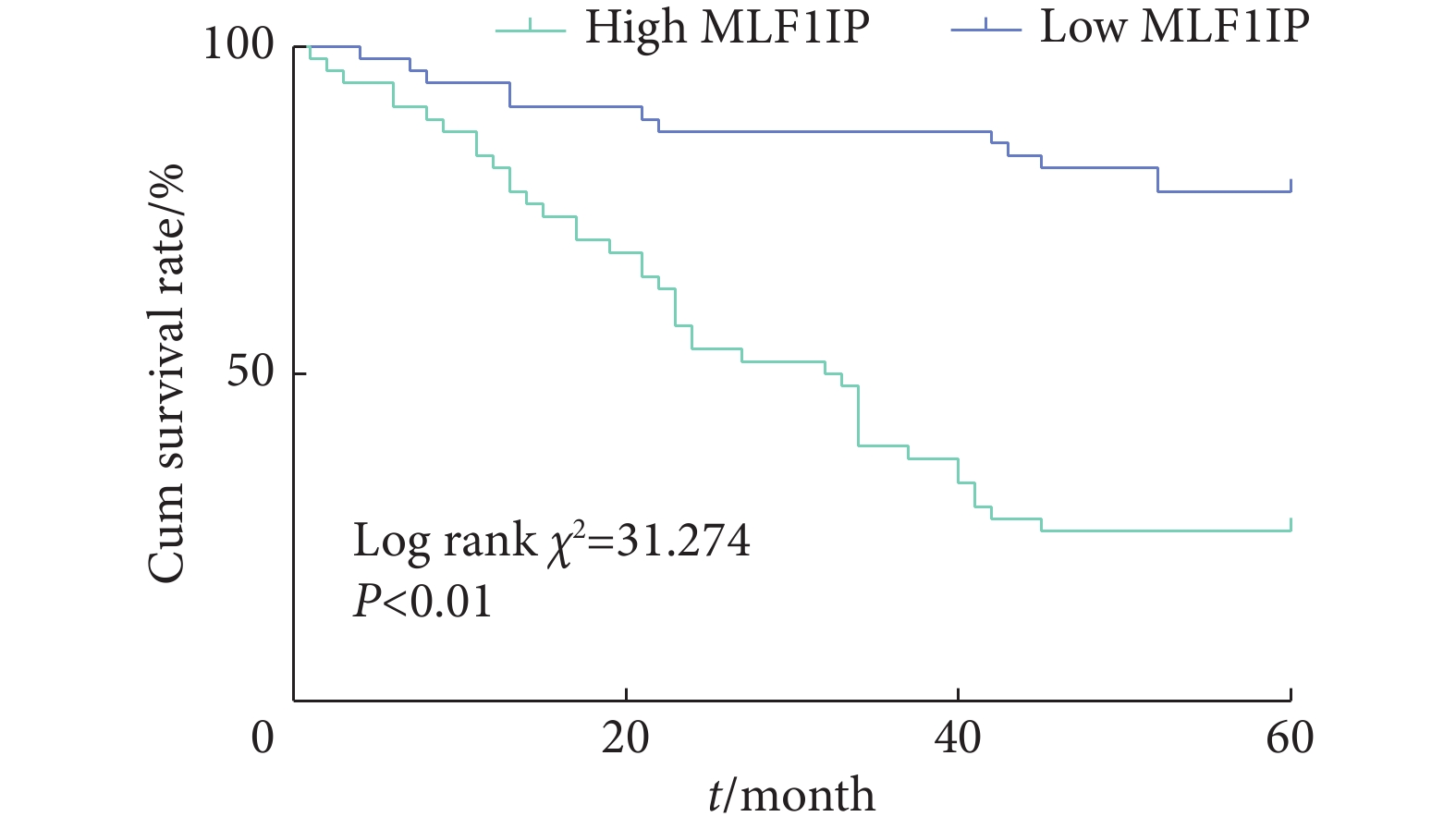
如图5,K-M生存分析显示,MLF1IP高表达组患者术后5年生存率低于MLF1IP低表达组(P<0.01)。
2.5 影响胃癌患者根治术后5年生存率的危险因素
如表2,单因素分析显示,胃癌组织中MLF1IP表达水平、外周血CEA≥5 μg/L、CA19-9≥37 kU/L、T分期为3~4期及N分期为2~3期是影响患者术后5年生存率的因素(P<0.05);进一步的Cox回归分析显示,以上因素均是影响胃癌患者术后5年生存率的独立危险因素(P<0.05)。
表 2 影响胃癌根治术后5年生存率的危险因素Table 2. Risk factors affecting 5-year survival rate after radical gastrectomy for gastric cancerCharacteristic Univariate analysis Multivariate analysis Log rank χ2 P HR 95% CI P Sex (male vs. female) 3.039 0.081 Age (<60 yr. vs ≥60 yr.) 0.000 0.992 MLF1IP expression (high vs. low) 31.274 <0.01 2.508 1.259-4.999 0.009 CEA (<5 μg/L vs. ≥5 μg/L) 18.654 <0.01 2.171 1.152-4.092 0.017 CA19-9 (<37 kU/L vs. ≥37 kU/L) 36.216 <0.01 2.401 1.094-5.269 0.029 Tumor size (<5 cm vs. ≥5 cm) 2.742 0.098 Histological type (adenocarcinoma vs. other) 0.005 0.943 T stage (T1-T2 vs. T3-T4) 24.790 <0.01 2.779 1.049-7.358 0.040 N stage (N0-N1 vs. N2-N3) 26.591 <0.01 2.072 1.100-3.904 0.024 HR: hazard ratio; CI: confidence interval. 2.6 MLF1IP表达量对胃癌患者根治术后5年生存的预测价值
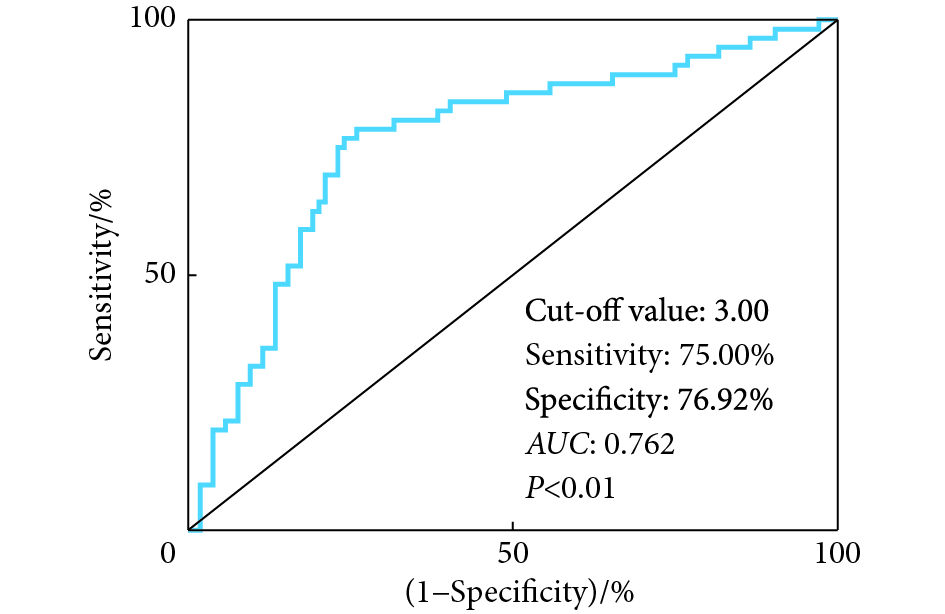
如图6,ROC曲线分析显示,以MLF1IP相对表达量预测胃癌患者术后5年生存的曲线下面积为0.762(P <0.01),以MLF1IP相对表达量3.00为截点值,敏感性为75.00%,特异性为76.92%。
2.7 干扰MLF1IP基因可抑制胃癌细胞的增殖、迁移和侵袭
采用慢病毒转染的方式构建低表达MLF1IP基因的MGC803细胞株。Western blot检测结果显示,敲低MLF1IP基因后,其蛋白表达水平较对照组降低(P<0.05),提示转染成功(图7A、7B)。进一步的CCK-8细胞实验显示,敲低MLF1IP基因可显著抑制胃癌细胞的增殖能力(P<0.05,图7C);Transwell实验结果显示,敲低MLF1IP基因对胃癌细胞迁移和侵袭能力均有抑制作用(P<0.05,图7D、7F、7G)。此外,划痕实验进一步显示,敲低MLF1IP基因后,胃癌细胞迁移能力较对照组降低(P<0.05,图7E、7H)。
![]() 图 7 敲低MLF1IP基因对胃癌细胞增殖、迁移和侵袭能力的影响Figure 7. Effects of MLF1IP gene knockdown on the proliferation, migration, and invasion of gastric cancer cellsA, B: MLF1IP protein expression in si-Control group and si-MLF1IP group was analyzed by Western blot (n=6); C: the proliferation ability of the cells was analyzed by CCK-8 (n=6); D: the migration and invasion ability of the cells were analyzed by Transwell assay; E: the migration ability of the cells was analyzed by scratch assay; F, G, H: quantitative analysis of the migration and invasion ability of the cells (n=6). * P<0.05, vs. si-Control.
图 7 敲低MLF1IP基因对胃癌细胞增殖、迁移和侵袭能力的影响Figure 7. Effects of MLF1IP gene knockdown on the proliferation, migration, and invasion of gastric cancer cellsA, B: MLF1IP protein expression in si-Control group and si-MLF1IP group was analyzed by Western blot (n=6); C: the proliferation ability of the cells was analyzed by CCK-8 (n=6); D: the migration and invasion ability of the cells were analyzed by Transwell assay; E: the migration ability of the cells was analyzed by scratch assay; F, G, H: quantitative analysis of the migration and invasion ability of the cells (n=6). * P<0.05, vs. si-Control.2.8 过表达MLF1IP基因可促进胃癌细胞增殖、迁移和侵袭
Western blot结果显示,过表达MLF1IP组中MLF1IP蛋白表达水平较对照组显著升高,提示转染成功(P<0.05,图8A、8B)。CCK-8细胞实验显示,过表达MLF1IP基因可显著增强胃癌细胞的增殖能力(P<0.05,图8C)。Transwell实验结果显示,过表达MLF1IP基因可显著增强胃癌细胞的迁移和侵袭能力(P<0.05,图8D、8F、8G)。同时划痕实验显示,过表达MLF1IP基因后,胃癌细胞迁移能力较对照组显著增强(P<0.05,图8E、8H)。
![]() 图 8 过表达MLF1IP基因对胃癌细胞的增殖、迁移和侵袭能力的影响Figure 8. Effects of the overexpression of MLF1IP gene on the proliferation, migration, and invasion of gastric cancer cellsA, B: The protein expression of MLF1IP in LV-Control group and LV-MLF1IP group was analyzed by Western bolt (n=6); C: cell proliferation ability was analyzed by CCK-8 assay (n=6); D: cell migration and invasion ability was analyzed by Transwell assay; E: cell migration ability was analyzed by scratch assay; F, G, H: quantitative analysis of the cell migration and invasion ability (n=6). * P<0.05, vs. LV-MLF1IP.
图 8 过表达MLF1IP基因对胃癌细胞的增殖、迁移和侵袭能力的影响Figure 8. Effects of the overexpression of MLF1IP gene on the proliferation, migration, and invasion of gastric cancer cellsA, B: The protein expression of MLF1IP in LV-Control group and LV-MLF1IP group was analyzed by Western bolt (n=6); C: cell proliferation ability was analyzed by CCK-8 assay (n=6); D: cell migration and invasion ability was analyzed by Transwell assay; E: cell migration ability was analyzed by scratch assay; F, G, H: quantitative analysis of the cell migration and invasion ability (n=6). * P<0.05, vs. LV-MLF1IP.2.9 干预MLF1IP基因对裸鼠成瘤能力的影响
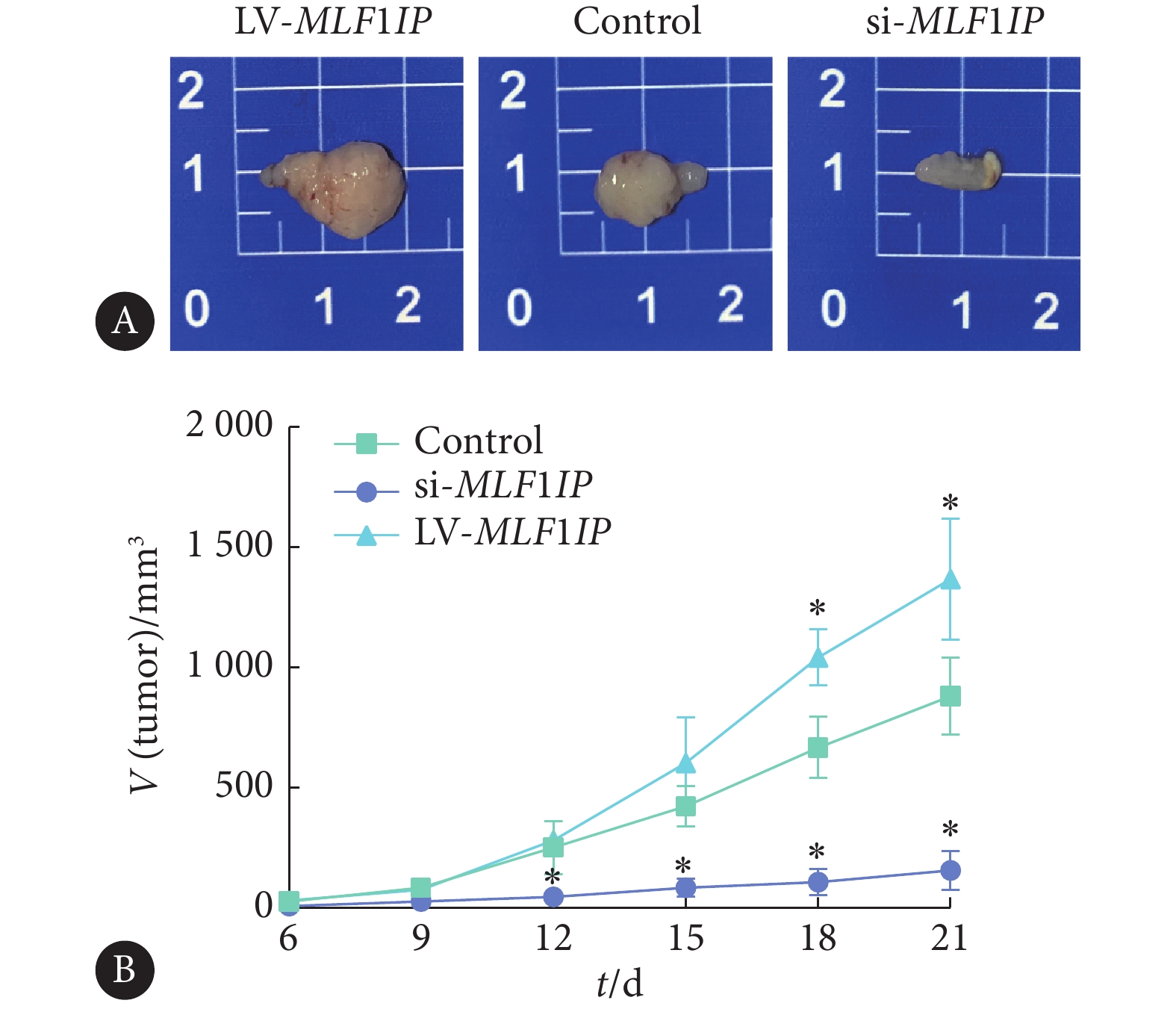
如图9所示,通过对裸鼠移植瘤体积进行组间比较发现,敲低MLF1IP基因组肿瘤体积较对照组缩小(P<0.05),而过表达MLF1IP基因组肿瘤体积较对照组增大(P<0.05)。
3. 讨论
胃癌发展速度快、侵袭性强及易转移,被发现时常常已是中晚期[8],且更为遗憾的是,目前临床上仍缺乏评估胃癌患者远期预后的有效指标[9]。既往研究表明,肿瘤大小、淋巴结转移、病理分期、TNM分期等均是影响患者术后5年生存率的重要因素,但仅靠这些指标评定患者的远期预后效果并不理想[10]。肿瘤标志物作为肿瘤细胞产生的特异性物质,也能作为反映胃癌进展状态的辅助诊断指标[11-12]。因此探索评估胃癌患者远期预后的临床生物指标对提高患者术后生存质量至关重要。MLF1LP又称KLIP1,是一种能够招募和激活细胞周期事件的关键调控因子,在细胞有丝分裂、中心体成熟等生理过程中发挥重要作用[13-14]。新近研究发现MLF1IP在多种恶性肿瘤中扮演了重要角色[15-18]。然而,MLF1IP对胃癌的发生发展及预后的影响尚未见报道,因此本研究尝试分析胃癌中MLF1IP表达的预后价值及其对肿瘤进展的调控作用,为胃癌的诊疗革新及预后评估提供新思路。
本研究通过GEO、Kaplan-Meier Plotter数据库显示MLF1IP在胃癌组织中显著高表达,且其高表达与患者预后不良相关。免疫组化染色显示MLF1IP在胃癌组织中表达上调,与生物信息学分析结果一致,提示MLF1IP可能参与了胃癌的进展。接下来,我们评估了MLF1IP表达量与患者临床病理参数之间的关系。数据分析显示,MLF1IP高表达组患者CEA≥5 μg/L、CA19-9≥37 kU/L、T分期为3~4期和N分期为2~3期的比例显著高于MLF1IP低表达组。相关性分析显示胃癌组织中MLF1IP与CEA、CA19-9的表达量均呈正相关关系。外周血中的CEA和CA19-9表达量被广泛运用于临床上胃癌的诊断和预后[12, 19],这提示MLF1IP可能同样具有作为肿瘤标志物的潜质。为了探寻MLF1IP在胃癌诊疗中的价值,本研究结合K-M生存分析及Cox回归模型证明,MLF1IP高表达与胃癌患者术后预后不良相关且可以作为影响胃癌患者远期预后的独立危险因素。进一步采用ROC曲线分析MLF1IP对胃癌患者术后5年生存期的预判价值,结果显示MLF1IP预测胃癌术后5年死亡的敏感性及特异性均大于70%,具有预后判断价值。
以上结果证明MLF1IP高表达与胃癌患者术后5年低生存率有关,但MLF1IP究竟以何种途径影响胃癌患者远期预后尚不清楚。肿瘤细胞增殖、迁移和侵袭等恶性生物学行为与癌症进展及患者预后密切相关[20],因此,本研究深入分析了MLF1IP对肿瘤细胞恶性生物学行为的影响,采用慢病毒转染的方式分别构建敲低和过表达MLF1IP基因的胃癌细胞株用于体内和体外研究。结果显示,敲低MLF1IP基因可显著抑制胃癌细胞的增殖、迁移和侵袭能力。同时,本研究建立了敲低和过表达MLF1IP基因小鼠模型,结果显示敲低MLF1IP基因显著抑制了肿瘤的生长,这与本研究细胞实验结果相符,进一步证实了MLF1IP可以调控肿瘤细胞的恶性行为。肿瘤的进展受多种分子及信号通路调控,既往研究报道MLF1IP可通过靶向BRCA1/AKT/p27信号促进结直肠癌细胞的增殖,参与疾病进展[17];此外,MLF1IP还被报道可通过调控CyclinD1,阻滞细胞周期G1,促进乳腺癌细胞的增殖[21]。因此,MLF1IP在其他肿瘤中的机制研究或许可以部分解释MLF1IP影响胃癌细胞恶性生物学行为的分子机制。本研究不仅是对MLF1IP生物学功能的进一步补充,同时为其可以作为评估胃癌患者远期预后潜在分子标志物提供了重要的实验依据。
综上,本研究证明MLF1IP在胃癌组织中表达升高并对患者预后不良有一定的预后价值,其可能参与了胃癌细胞增殖、迁移和侵袭等恶性行为。但其具体的分子机制尚未明确,还需要进一步地深入研究。
* * *
利益冲突 所有作者均声明不存在利益冲突
-
图 2 胃癌及癌旁组织中MLF1IP及Ki67的表达情况
Figure 2. Expression of MLF1IP and Ki67 in gastric cancer tissue and the adjacent tissue
A, B: Immunohistochemical staining of MLF1IP in gastric cancer tissue (×100, A) and the adjacent tissue (×400, B); C, D: immunohistochemical staining of Ki67 in gastric cancer tissue (×100, C) and the adjacent tissue (×400, D).
图 7 敲低MLF1IP基因对胃癌细胞增殖、迁移和侵袭能力的影响
Figure 7. Effects of MLF1IP gene knockdown on the proliferation, migration, and invasion of gastric cancer cells
A, B: MLF1IP protein expression in si-Control group and si-MLF1IP group was analyzed by Western blot (n=6); C: the proliferation ability of the cells was analyzed by CCK-8 (n=6); D: the migration and invasion ability of the cells were analyzed by Transwell assay; E: the migration ability of the cells was analyzed by scratch assay; F, G, H: quantitative analysis of the migration and invasion ability of the cells (n=6). * P<0.05, vs. si-Control.
图 8 过表达MLF1IP基因对胃癌细胞的增殖、迁移和侵袭能力的影响
Figure 8. Effects of the overexpression of MLF1IP gene on the proliferation, migration, and invasion of gastric cancer cells
A, B: The protein expression of MLF1IP in LV-Control group and LV-MLF1IP group was analyzed by Western bolt (n=6); C: cell proliferation ability was analyzed by CCK-8 assay (n=6); D: cell migration and invasion ability was analyzed by Transwell assay; E: cell migration ability was analyzed by scratch assay; F, G, H: quantitative analysis of the cell migration and invasion ability (n=6). * P<0.05, vs. LV-MLF1IP.
表 1 108例胃癌患者的基线资料
Table 1 The baseline data of 108 patients with gastric cancer
Characteristic n MLF1IP expression P Low (n=54) High (n=54) Sex/case (%) 0.837 Male 73 37 (50.7) 36 (49.3) Female 35 17 (48.6) 18 (51.4) Age/case (%) 0.077 <60 yr. 65 28 (43.1) 37 (56.9) ≥60 yr. 43 26 (60.5) 17 (39.5) CEA/case (%) <0.01 <5 μg/L 52 34 (65.4) 18 (34.6) ≥5 μg/L 56 20 (35.7) 36 (64.3) CA19-9/case (%) <0.01 <37 kU/L 51 40 (78.4) 11 (21.6) ≥37 kU/L 57 14 (24.6) 43 (75.4) Tumor size/case (%) 0.053 <5 cm 60 35 (58.3) 25 (41.7) ≥5 cm 48 19 (39.6) 29 (60.4) Histological type/case (%) 0.139 Adenocarcinoma 95 45 (47.4) 50 (52.6) Other 13 9 (69.2) 4 (30.8) T stage/case (%) <0.01 1-2 37 28 (75.7) 9 (24.3) 3-4 71 26 (36.6) 45 (63.4) N stage/case (%) <0.01 0-1 59 37 (62.7) 22 (37.3) 2-3 49 17 (34.7) 32 (65.3) 表 2 影响胃癌根治术后5年生存率的危险因素
Table 2 Risk factors affecting 5-year survival rate after radical gastrectomy for gastric cancer
Characteristic Univariate analysis Multivariate analysis Log rank χ2 P HR 95% CI P Sex (male vs. female) 3.039 0.081 Age (<60 yr. vs ≥60 yr.) 0.000 0.992 MLF1IP expression (high vs. low) 31.274 <0.01 2.508 1.259-4.999 0.009 CEA (<5 μg/L vs. ≥5 μg/L) 18.654 <0.01 2.171 1.152-4.092 0.017 CA19-9 (<37 kU/L vs. ≥37 kU/L) 36.216 <0.01 2.401 1.094-5.269 0.029 Tumor size (<5 cm vs. ≥5 cm) 2.742 0.098 Histological type (adenocarcinoma vs. other) 0.005 0.943 T stage (T1-T2 vs. T3-T4) 24.790 <0.01 2.779 1.049-7.358 0.040 N stage (N0-N1 vs. N2-N3) 26.591 <0.01 2.072 1.100-3.904 0.024 HR: hazard ratio; CI: confidence interval. -
[1] TANG B, YANG S. Involvement of heparanase in gastric cancer progression and immunotherapy. Adv Exp Med Biol,2020,1221: 351–363. DOI: 10.1007/978-3-030-34521-1_13
[2] Van CUTSEM E, SAGAERT X, TOPAL B, et al. Gastric cancer. Lancet,2016,388(10060): 2654–2664. DOI: 10.1016/S0140-6736(16)30354-3
[3] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin,2018,68(6): 394–424. DOI: 10.3322/caac.21492
[4] NGUYEN P H, GIRAUD J, CHAMBONNIER L, et al. Characterization of biomarkers of tumorigenic and chemoresistant cancer stem cells in human gastric carcinoma. Clin Cancer Res,2017,23(6): 1586–1597. DOI: 10.1158/1078-0432.CCR-15-2157
[5] FENG G, ZHANG T, LIU J, et al. MLF1IP promotes normal erythroid proliferation and is involved in the pathogenesis of polycythemia vera. FEBS Lett,2017,591(5): 760–773. DOI: 10.1002/1873-3468.12587
[6] HUANG D P, LUO R C. MLF1IP is correlated with progression and prognosis in luminal breast cancer. Biochem Biophys Res Commun,2016,477(4): 923–926. DOI: 10.1016/j.bbrc.2016.06.159
[7] PAN T, ZHOU D, SHI Z, et al. Centromere protein U (CENPU) enhances angiogenesis in triple-negative breast cancer by inhibiting ubiquitin-proteasomal degradation of COX-2. Cancer Lett,2020,482: 102–111. DOI: 10.1016/j.canlet.2019.11.003
[8] De MANZONI G, YANG H K. Gastric cancer surgery. Updates Surg,2018,70(2): 155. DOI: 10.1007/s13304-018-0551-3
[9] SUN J, SHEN D, ZHENG Y, et al. USP8 inhibitor suppresses HER-2 positive gastric cancer cell proliferation and metastasis via the PI3K/AKT signaling pathway. Onco Targets Ther,2020,13: 9941–9952. DOI: 10.2147/OTT.S271496
[10] 葛思堂, 王姗, 项武军, 等. 腺苷酸环化酶相关蛋白2在胃癌组织中高表达并与肿瘤进展密切相关. 南方医科大学学报,2019,39(9): 1052–1058. DOI: 10.12122/j.issn.1673-4254.2019.09.08 [11] LAZĂR D C, AVRAM M F, ROMOȘAN I, et al. Prognostic significance of tumor immune microenvironment and immunotherapy: novel insights and future perspectives in gastric cancer. World J Gastroenterol,2018,24(32): 3583–3616. DOI: 10.3748/wjg.v24.i32.3583
[12] FENG F, TIAN Y, XU G, et al. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer,2017,17(1): 737. DOI: 10.1186/s12885-017-3738-y
[13] WANG X, MARCINKIEWICZ M, GATAIN Y, et al. Investigation of tissue-specific expression and functions of MLF1-IP during development and in the immune system. PLoS One,2013,8(5): e63783. DOI: 10.1371/journal.pone.0063783
[14] HANISSIAN S H, AKBAR U, TENG B, et al. cDNA cloning and characterization of a novel gene encoding the MLF1-interacting protein MLF1IP. Oncogene,2004,23(20): 3700–3707. DOI: 10.1038/sj.onc.1207448
[15] HANISSIAN S H, TENG B, AKBAR U, et al. Regulation of myeloid leukemia factor-1 interacting protein (MLF1IP) expression in glioblastoma. Brain Res,2005,1047(1): 56–64. DOI: 10.1016/j.brainres.2005.04.017
[16] YU Q L, JUN L, YAN W Z, et al. The centromere-associated protein CENPU promotes cell proliferation, migration, and invasiveness in lung adenocarcinoma. Cancer Lett,2022,532: 215599. DOI: 2022.53210.1016/j.canlet.2022.215599
[17] XU Y, ZHANG L, WANG Q, et al. Overexpression of MLF1IP promotes colorectal cancer cell proliferation through BRCA1/AKT/p27 signaling pathway. Cell Signal,2022,92: 110273. DOI: 10.1016/j.cellsig.2022.110273
[18] 黄督平. MLF1IP在Luminal型乳腺癌中的表达及其对他莫昔芬敏感性的影响. 广州: 南方医科大学, 2017. [19] SHIBATA C, NAKANO T, YASUMOTO A, et al. Comparison of CEA and CA19-9 as a predictive factor for recurrence after curative gastrectomy in gastric cancer. BMC Surg,2022,22(1): 213. DOI: 10.1186/s12893-022-01667-z
[20] DUFF D, LONG A. Roles for RACK1 in cancer cell migration and invasion. Cell Signal,2017,35: 250–255. DOI: 10.1016/j.cellsig.2017.03.005
[21] YANG F, WANG Y H, DONG S Y, et al. MLF1IP promotes cells proliferation and apoptosis by regulating CyclinD1 in breast cancer. Int J Clin Exp Pathol,2017,10(12): 11554–11562.
-
期刊类型引用(9)
1. 赵皓,张文静,杨子,张小凤,牛嘉琪,宋雪. CABYR在胃癌组织中的异常表达及其临床意义. 中华全科医学. 2025(01): 40-44 .  百度学术
百度学术
2. 南静. MLF1IP在前列腺癌组织中表达及临床意义. 航空航天医学杂志. 2025(02): 155-158 .  百度学术
百度学术
3. 张保根,杨澍,陈斌妮,陈文,郑梓莹,张燕. 孕激素相关子宫内膜蛋白在胃癌早期诊断、预后中的作用. 四川大学学报(自然科学版). 2024(01): 192-199 .  百度学术
百度学术
4. 张诺,张震,张雨路,宋雪,张小凤,李静,左芦根,胡建国. PCID2在胃癌组织中高表达并通过调控细胞周期进程和增殖影响患者预后. 南方医科大学学报. 2024(02): 324-332 .  百度学术
百度学术
5. 袁志鹏,郑锐年,袁淑卿,陈镜塘,吴依芬. 胃癌组织miR-597、miR-3188表达与患者临床病理特征及预后的关系. 国际检验医学杂志. 2024(08): 976-980 .  百度学术
百度学术
6. 张宗兵,乐正宏,刘牧林,郝博. ITGB2在胃癌中的表达及其临床价值. 中华全科医学. 2024(07): 1102-1107 .  百度学术
百度学术
7. 张震,鲁辉,陈孝华,王炼,王子良,王月月,葛思堂,左芦根. CEP192过表达可作为胃癌患者不良预后的生物标志物并通过调控G2/M期关键蛋白的表达影响肿瘤细胞恶性增殖. 南方医科大学学报. 2024(11): 2137-2145 .  百度学术
百度学术
8. 林洁,周晓黎,林静. 胃蛋白酶原、胃泌素17联合~(13)C尿素呼气试验在早期胃癌筛查中的应用. 保健医学研究与实践. 2023(08): 53-57 .  百度学术
百度学术
9. 李杰,侯玮,张燕,常青. MLF1IP、SIRT6在胃癌组织中的表达及意义. 临床肿瘤学杂志. 2023(10): 920-923 .  百度学术
百度学术
其他类型引用(1)

开放获取 本文遵循知识共享署名—非商业性使用4.0国际许可协议(CC BY-NC 4.0),允许第三方对本刊发表的论文自由共享(即在任何媒介以任何形式复制、发行原文)、演绎(即修改、转换或以原文为基础进行创作),必须给出适当的署名,提供指向本文许可协议的链接,同时标明是否对原文作了修改;不得将本文用于商业目的。CC BY-NC 4.0许可协议详情请访问 https://creativecommons.org/licenses/by-nc/4.0

 首页
首页


 下载:
下载: