Effect and Mechanism of Treating Experimental Autoimmune Encephalomyelitis in Mice with Butylphthalide Combined with Bone Marrow Mesenchymal Stem Cells
-
摘要:目的 探索丁苯 探索丁苯酞(3-n-butylphthalide, NBP)和骨髓间充质干细胞(bone mesenchymal stem cells, BMSCs)联合治疗小鼠实验性自身免疫性脑脊髓炎(experimental autoimmune encephalomyelitis, EAE)的疗效和机制。方法 用髓鞘少突胶质糖蛋白(myelin oligodendrocyte glycoprotein 35-55, MOG35-55)诱导C57BL/6小鼠建立EAE模型,将小鼠随机分为EAE组〔腹腔注射磷酸盐缓冲生理盐水 (phosphate-buffered saline, PBS)〕、NBP处理EAE组(NBP组,腹腔注射NBP)、BMSCs移植EAE组(BMSCs组,BMSCs注入侧脑室,腹腔注射PBS)、BMSCs和NBP联合处理EAE组(BMSCs+NBP组,BMSCs注入侧脑室,腹腔注射NBP),每组各10只。并设正常小鼠10只为空白对照组(腹腔注射PBS)。每日记录小鼠神经功能评分。诱导EAE 22 d后处死小鼠,劳克坚劳蓝(Luxol fast blue, LFB)脊髓髓鞘染色观察脱髓鞘情况;酶联免疫吸附法(enzyme-linked immunosorbent assay, ELISA)检测血清白介素(interleukin, IL)-6、IL-10、IL-17、IL-22和转化生长因子-β(transforming growth factor-β, TGF-β)水平;免疫荧光染色检测脑中胶质纤维酸性蛋白(glial fibrillary acidic protein, GFAP)、微管相关蛋白-2(microtubule-associated protein-2, MAP-2)、髓鞘碱性蛋白(myelin basic protein, MBP)的表达;Western blot法检测脊髓核因子κB〔nuclear factor (NF)-κB〕通路、磷脂酰肌醇-3激酶(phosphoinositide-3 kinase, PI3K)/蛋白激酶(protein kinase B, PKB or Akt)通路及IL-17、叉状头/翅膀状螺旋转录因子(forkhead box P3, Foxp3)的表达。结果 各治疗组神经功能评分及平均分均较EAE组降低(P<0.05);BMSCs+NBP组评分下降较单独治疗组(NBP组和BMSCs组)更为明显(P<0.05);LFB染色与神经功能评分及平均分一致。与EAE组相比,各治疗组EAE小鼠血清促炎因子IL-6、IL-17和IL-22降低(P<0.05),抑炎因子IL-10和TGF-β增加(P<0.05);BMSCs+NBP组细胞因子表达的变化较BMSCs组更为显著(P<0.05)。BMSCs+NBP组的GFAP、MAP-2、MBP表达均较BMSCs组增加(P<0.05)。与EAE组相比,各治疗组p-NF-κB/NF-κB比值下降,而p-IκBα/IκBα比值上升,p-PI3K/PI3K及p-Akt/Akt比值均上升,IL-17/Foxp3比值降低,以BMSCs+NBP组最为显著(P<0.05)。结论 NBP和BMSCs联合治疗可缓解EAE模型小鼠的症状,且疗效优于单独治疗。其机制与抑制NF-κB通路以调节Th17/Foxp3比例及激活PI3K/Akt通路以促进BMSCs成神经分化有关。
-
关键词:
- 实验性自身免疫性脑脊髓炎 /
- 骨髓间充质干细胞 /
- 丁苯酞 /
- NF-κB通路 /
- PI3K/Akt通路
Abstract:Objective To explore the efficacy and mechanism of using 3-n-butylphthalide (NBP) in combination with bone marrow mesenchymal stem cells (BMSCs) in the treatment of experimental autoimmune encephalomyelitis (EAE) in mice.Methods Myelin oligodendrocyte glycoprotein (MOG35-55) was used for the induction and establishment of the EAE model in C57BL/6 mice. The mice were randomly assigned to the EAE group, which received intraperitoneal injection of phosphate-buffered saline (PBS), the NBP-treated EAE group, or the NBP group, which received intraperitoneal injection of NBP, the BMSCs transplantion EAE group, or the BMSCs group, which received BMSCs injected into the lateral ventricle and intraperitoneal injection of PBS, and the BMSCs and NBP combination treatment EAE group, or the BMSCs+NBP group, which received BMSCs injected into the lateral ventricle and intraperitoneal injection of NBP. Each group had 10 mice, while ten normal mice were used as the blank control group receiving intraperitoneal injection of PBS. The neurological function scores were documented daily. The mice were sacrificed 22 days after EAE induction, and the demyelination state of of the spinal cords was observed through Luxol fast blue (LFB) staining. In addition, the levels of serum interleukin-6 (IL-6), IL-10, IL-17, IL-22 and transforming growth factor-β (TGF-β) were examined with ELISA. The levels of glial fibrillary acidic protein (GFAP), microtubule associated protein-2 (MAP-2) and myelin basic protein (MBP) in the brain were examined with immunofluorescence staining. Western blot was used to check the expressions of nuclear factor (NF)-κB pathway, phosphoinositide-3 kinase (PI3K)/protein kinase B (PKB or Akt) pathway, IL-17 and forkhead box P3 (Foxp3) in the spinal cords.Results The neurological function scores and average scores of each treatment group were significantly lower than those of the EAE group (P<0.05). The scores of the BMSCs+NBP group decreased more significantly than those of the single treatment groups (the NBP group and the BMSCs group) (P<0.05). LFB staining results of the spinal cords were consistent with the neurological function scores and the average scores. Compared with the EAE group, the levels of pro-inflammatory cytokines, including IL-6, IL-17 and IL-22, significantly decreased (P<0.05), and the levels of anti-inflammatory cytokines IL-10 and TGF-β significantly increased (P<0.05). The change in cytokine expression was more significant in the BMSCs+NBP group (P<0.05). The expressions of GFAP, MAP-2 and MBP in the BMSCs+NBP group were significantly higher than those of the BMSCs group (P<0.05). Compared with the EAE group, the p-NF-κB/NF-κB ratio and the IL-17/Foxp3 ratio in NBP group, BMSCs group and BMSCs+NBP group decreased, while P-IκBα/IκBα, p-pI3k/PI3K and P-Akt/Akt ratios increased, especially in the BMSCs+NBP group(P<0.05).Conclusion The combined treatment of NBP and BMSCs can help alleviate the symptoms of EAE model mice, showing better efficacy than treatment with NBP or BMSCs alone. The mechanism is related to the inhibition of the NF-κB pathway to regulate Th17/Foxp3 ratio and the activation of the PI3K/Akt pathway to promote the neurogenic differentiation of BMSCs. -
多发性硬化症(multiplesclerosis, MS)是一种以炎症、脱髓鞘和变性为特征的中枢神经系统自身免疫性疾病[1]。迄今,还没有阻止神经轴突损伤和促进髓鞘再生的有效方法[1]。实验性自身免疫性脑脊髓炎(experimental autoimmune encephalomyelitis, EAE)模型是最常用的MS动物模型[2]。骨髓间充质干细胞(bone mesenchymal stem cells, BMSCs)已被用作治疗EAE/MS的“种子细胞”[3]。BMSCs可降低EAE小鼠白介素(IL)-17、并提升转化生长因子-β(TGF-β)含量[4],调节T辅助细胞(Th)17细胞/T调节(Treg)细胞平衡,起到一定免疫调节作用。另外,给EAE模型静脉注射BMSCs,BMSCs可分化为多种神经细胞[5],起到部分神经修复作用。然而,单独干细胞移植EAE的抗炎效果有限,成神经分化率偏低成为制约其应用的瓶颈,因此寻找更有效的联合治疗方法成为当务之急。丁苯酞(3-n-butylphthalide, NBP)是从芹菜种子中分离出的一种化合物,已用于治疗急性缺血性中风[6]。NBP的抗炎作用也已得到证实[7]。本研究从免疫调节、促进干细胞成神经分化的角度探索NBP和BMSCs联合治疗EAE的效果和机制,为NBP和BMSCs联合用于临床治疗MS提供依据。
1. 材料和方法
1.1 主要材料和仪器
已转染绿色荧光蛋白 (GFP)的人BMSCs(上海百利生物技术有限公司);胎牛血清(美国Gibco);DMEM/F12培养基(美国Gibco);CD29、CD34、CD45和CD90抗体(美国Abcam);MOG35-55(上海吉尔生化有限公司);百日咳毒素(PTX)和弗氏完全佐剂(CFA)(美国Sigma);灭活H37RA结核分枝杆菌(美国BD);小鼠IL-6、IL-10、IL-17、IL-22和TGF-β ELISA试剂盒(美国Abcam);兔抗小鼠胶质纤维酸性蛋白(GFAP)、微管相关蛋白-2(MAP-2)、髓鞘碱性蛋白(MBP)抗体(一抗)(美国Abcam);山羊抗兔荧光二抗(美国KPL);劳克坚劳蓝(LFB)髓鞘染色液(上海哈灵生物);兔抗小鼠核因子κB(NF-κB)、磷酸化NF-κB(p-NF-κB)、NF-κB抑制蛋白α(IκBα)、磷酸化IκBα(p-IκBα)、丝氨酸-苏氨酸特异性蛋白激酶(Akt)、磷酸化Akt(p-Akt)、磷脂酰肌醇-3激酶(PI3K)、磷酸化PI3K(p-PI3K)、IL-17、叉状头/翅膀状螺旋转录因子(Foxp3)抗体(一抗)和辣根过氧化物酶标记山羊抗兔IgG抗体(二抗)(美国CST)。
荧光显微镜(日本奥林巴斯);酶联免疫吸附板阅读器(美国Molecular Devices);流式细胞仪(德国Millipore),全自动紫外与可见分析装置-凝胶成像系统(上海天能)。
1.2 实验方法
1.2.1 人BMSCs的培养及鉴定
在37 ℃和体积分数5% CO2条件下,用含10%胎牛血清的DMEM/F12培养基在恒温细胞培养箱里培养BMSCs并传代。取第3代细胞,倒置显微镜及荧光显微镜观察BMSCs的生长状态及形态,流式细胞术检测BMSCs的CD29、CD34、CD45和CD90分子的表达。后继实验采用第3~5代BMSCs进行移植。
1.2.2 EAE小鼠模型的制备、处理分组
8周龄雌性C57BL/6小鼠购自昆明医科大学。饲养于昆明医科大学实验动物中心SPF级动物房,光/暗周期为12 h/12 h,24~26 ℃,可自由获得食物和饮水。所有实验均得到昆明医科大学伦理委员会批准,并按照昆明医科大学伦理委员会的规定进行。MOG35-55用磷酸盐缓冲生理盐水 (Phosphate-buffered saline, PBS)(pH=7.2)稀释至0.01 mol/mL,并与含灭活H37RA结核分枝杆菌的CFA混合,充分乳化混匀,用来诱导EAE模型。每只小鼠背部皮下注射以上混合物150 μL,分三点注射。造模用时3 d:诱导EAE模型当天,小鼠背部皮肤三点注射上述混合物,然后腹腔注射PTX 200 ng,第3天腹腔给予相同剂量的PTX。第1天用药后,开始每天监测小鼠的神经功能评分,直至动物处死。一旦当日出现神经功能评分≥0.5分(具体见1.2.3),则判定造模成功。空白对照组模拟EAE诱导过程:第1天予以生理盐水150 μL背部皮肤注射,腹腔注射生理盐水100 μL;第3天腹腔注射生理盐水100 μL。50只小鼠随机分为5组(n=10):空白对照组、EAE组、NBP处理EAE组(NBP组)、BMSCs移植EAE组(BMSCs组)、BMSCs和NBP联合处理EAE组(BMSCs+NBP组)。除空白对照组外,其余4组均选用EAE造模成功的小鼠。在BMSCs组或BMSCs+NBP组第1只小鼠出现症状(鼠尾瘫痪、不能竖起等)后,通过脑立体定向技术将BMSCs细胞(2×105个BMSCs,体积为2 μL)注入这两组EAE小鼠侧脑室。同时,对NBP组和BMSCs+NBP组的EAE小鼠腹腔注射NBP(80 mg/kg),空白对照组及其余EAE小鼠腹腔注射相同体积PBS,每日1次。
1.2.3 神经功能评分
每日同时刻观察小鼠发病情况,同时采用双盲法记录神经功能评分。EAE模型评分标准为:0分,正常;0.5分,尾尖点地;1分,尾部下垂或轻度步态不稳;1.5分,尾巴完全拖地,步态不稳;2分,双后肢无力,或一肢瘫痪,被动翻身后可恢复;3分,双后肢瘫痪,被动翻身后不可恢复,但给予刺激后可挪动;4分,双后肢瘫痪,前肢瘫痪或肌力减弱伴尿便失禁;5分,死亡[8]。记录每日评分,绘图时采用偶数天的评分绘制,于第22日进行组间比较。计算和比较每组从第10天至第22天的平均分。诱导EAE 22 d后,麻醉后采用割尾静脉取血法,从小鼠身上采集血清,多聚甲醛心脏灌流固定,取脊髓和脑进行以下实验。
1.2.4 劳克坚劳蓝(Luxol fast blue, LFB)染色及病理评分
诱导EAE 22 d后,取各组小鼠脊髓,石蜡包埋并切片。小鼠脊髓组织石蜡切片置于二甲苯溶液中进行梯度水合。将切片置于50~65 ℃恒温箱中过夜。取染色切片,通过酒精、水,然后加入分色液。分化后用水冲洗3次,复染或梯度脱水。光镜下观察组织病理变化并进行评分。LFB评分标准:0分,无脱髓鞘;1分,罕见脱髓鞘;2分,少数脱髓鞘;3分,大面积脱髓鞘。
1.2.5 酶联免疫吸附试验(ELISA)检测小鼠血清的炎性因子
诱导EAE 22 d后,采集小鼠血清,ELISA法检测各组小鼠血清中IL-6、IL-10、IL-17、IL-22和TGF-β的水平。根据ELISA试剂盒说明书,匀浆后4 ℃、5 000 r/min、离心15 min,收集上清液。用ELISA试剂盒检测IL-6、IL-10、IL-17、IL-22和TGF-β的水平。用标准样品构造标准曲线,在450 nm处使用酶联免疫吸附板阅读器对ELISA结果进行量化。
1.2.6 免疫荧光染色检测小鼠脑组织神经细胞标志物的表达
诱导EAE 22 d后,取小鼠脑组织,加入含0.4% TritonX-100的PBS,室温下用5%牛血清白蛋白封闭脑组织1 h,GFAP、MAP-2、MBP一抗(1∶100)4 ℃孵育过夜。荧光二抗(1∶1 000)4 ℃孵育1 h,然后用DAPI染细胞核。用荧光显微镜捕捉图像,计算BMSCs组和BMSCs+NBP组EAE小鼠脑内BMSCs分化为神经元、星形胶质细胞和少突胶质细胞的数量。
1.2.7 Western blot法测定脊髓组织NF-κB信号通路、PI3K/Akt信号通路蛋白表达水平
诱导EAE 22 d后,取小鼠脊髓,−80 ℃保存。每个样品加300 μL蛋白裂解液,匀浆后离心(4 ℃,12 000 r/min,15 min),收集上清液。用BCA蛋白试剂盒测定蛋白浓度。取30 μg蛋白上样,10% SDS-PAGE电泳,蛋白分离后转膜至PDVF膜。5%脱脂奶粉封闭1.5 h,然后加一抗(1∶1 000)后4 ℃孵育过夜;次日,PBST洗涤3次,加二抗(1∶5 000)后室温孵育1 h,再用PBST洗涤3次;最后,用ECL显色试剂盒进行显色。用Image J分析蛋白条带积分吸光度,以目标蛋白与内参蛋白β-actin积分吸光度比值表示其相对表达水平。然后计算p-PI3K/PI3K比值、p-Akt/Akt比值、IL-17/Foxp3比值。
1.3 统计学方法
实验结果数据均用
$ \bar x \pm s $ 表示。多组间比较经单因素方差分析(one-way ANOVA)检验,两组间比较采用t检验,P<0.05为差异有统计学意义。2. 结果
2.1 BMSCs形态和鉴定结果
用倒置显微镜(图1A)和荧光显微镜(图1B)观察培养的BMSCs,可见BMSCs贴壁,呈多边形形态。BMSCs生长到第3代后,其形态类似成纤维细胞。流式细胞术检测BMSCs表面标志物的表达(图1C~1F),结果表明,培养的BMSCs高表达CD29(95.2%)和CD90(94.13%),而低表达CD34(1.27%)和CD45(0.92%),符合BMSCs的分子表型。
![]() 图 1 人骨髓间充质干细胞的形态和表面标记物的表达Figure 1. Human BMSCs morphology and surface marker expressionBMSCs were attached to a polygonal form. After BMSCs grew to the third generation, their shape became similar to that of fibroblasts (×100) in light microscope (A) and fluorescence microscope (B); C-F: CD29, CD34, CD45 and CD90 surface marker expression was determined by flow cytometry. The result showed that cultured BMSCs expressed CD29 and CD90, while there was no expression of CD34 and CD45 on the surface of BMSCs. R1: Regin 1; RN1: Positive fluorescence regin.
图 1 人骨髓间充质干细胞的形态和表面标记物的表达Figure 1. Human BMSCs morphology and surface marker expressionBMSCs were attached to a polygonal form. After BMSCs grew to the third generation, their shape became similar to that of fibroblasts (×100) in light microscope (A) and fluorescence microscope (B); C-F: CD29, CD34, CD45 and CD90 surface marker expression was determined by flow cytometry. The result showed that cultured BMSCs expressed CD29 and CD90, while there was no expression of CD34 and CD45 on the surface of BMSCs. R1: Regin 1; RN1: Positive fluorescence regin.2.2 NBP联合BMSCs移植治疗对EAE小鼠神经功能和EAE脱髓鞘病变的影响
见图2。空白对照组神经功能评分每时点均为0分。NBP组、BMSCs组和BMSCs+NBP组小鼠神经功能评分及平均分均较EAE组降低(P<0.05);NBP组和BMSCs组两组间比较差异无统计学意义(P>0.05)。与NBP组和BMSCs组比较,BMSCs+NBP组评分下降(P<0.01)。小鼠脊髓LFB染色结果与神经功能评分及平均分一致。与空白对照组相比,EAE组脊髓组织白质区有大面积的髓鞘脱失,其病理学评分也高于空白对照组(P<0.001)。单独应用NBP或BMSCs移植治疗,脱髓鞘面积均有所缩小,但两组病理学评分之间无明显差别(P>0.05)。在BMSCs+NBP组,EAE小鼠的脱髓鞘面积较单独治疗组缩小,病理学评分与与NBP组和BMSCs组比较差异均有统计学意义(P<0.05或P<0.01),与EAE组比较差异有统计学意义(P<0.001)。以上结果提示,NBP联合BMSCs移植可明显改善EAE小鼠的神经功能和髓鞘脱失,且优于单独应用NBP或者BMSCs移植治疗。见图3。
![]() 图 3 NBP联合BMSCs移植治疗对EAE小鼠脱髓鞘病变的影响。 LFB染色 ×400Figure 3. Effect of NBP combined with BMSCs transplantation on demyelinating lesions in EAE mice. LFB staining ×400A: Control group, no demyelination; B: EAE group, large areas of demyelination (black arrows); C: NBP group, after NBP treatment, compared with the EAE group, there was significantly less demyelination (black arrows); D: BMSCs group, after BMSCs transplantation, the area of demyelination (black arrows) was significantly reduced compared with that of the EAE group; E: BMSCs+NBP group, after combined treatment with NBP and BMSCs, compared with EAE group, demyelination was greatly reduced; compared with NBP and BMSCs alone treatment group, demyelination was further reduced (black arrows). F: Pathological score, n=10, *P<0.05, **P<0.01, *** P<0.001, ****P<0.000 1.
图 3 NBP联合BMSCs移植治疗对EAE小鼠脱髓鞘病变的影响。 LFB染色 ×400Figure 3. Effect of NBP combined with BMSCs transplantation on demyelinating lesions in EAE mice. LFB staining ×400A: Control group, no demyelination; B: EAE group, large areas of demyelination (black arrows); C: NBP group, after NBP treatment, compared with the EAE group, there was significantly less demyelination (black arrows); D: BMSCs group, after BMSCs transplantation, the area of demyelination (black arrows) was significantly reduced compared with that of the EAE group; E: BMSCs+NBP group, after combined treatment with NBP and BMSCs, compared with EAE group, demyelination was greatly reduced; compared with NBP and BMSCs alone treatment group, demyelination was further reduced (black arrows). F: Pathological score, n=10, *P<0.05, **P<0.01, *** P<0.001, ****P<0.000 1.2.3 各组小鼠血清IL-6、IL-17、IL-22、IL-10和TGF-β水平的变化
ELISA结果(表1)显示:与空白对照组相比,EAE组小鼠血清促炎因子(IL-6、IL-17和IL-22)增加,抑炎因子(IL-10和TGF-β)降低(P<0.05)。与EAE组相比,NBP组、BMSCs组及BMSCs+NBP组EAE小鼠血清促炎因子水平下降,而抑炎因子水平提升(P<0.05);联合治疗组(BMSCs+NBP组)与单独移植组(BMSCs组)相比,促炎因子水平都进一步降低,与之相反,抑炎因子均进一步增加(P<0.05)。
表 1 各组小鼠血清IL-6、IL-17、IL-22、IL-10和TGF-β水平的变化Table 1. Changes of serum levels of IL-6, IL-17, IL-22, IL-10 and TGF-β in the mice of each groupIndex Group Control (n=10) EAE (n=10) NBP (n=10) BMSCs (n=10) BMSCs+NBP (n=10) IL-6/(pg/mL) 91.1±18.5 188.1±35.0# 145.1±17.9△ 132.6±19.6△△ 101.3±21.6*, △△ IL-17/(pg/mL) 50.4±9.1 120.6±10.5# 84.0±6.2△ 74.4±7.7△△ 60.4±8.1*, △△ IL-22/(pg/mL) 223.5±80.6 515.1±58.6# 402.0±77.9△ 339.8±89.0△△ 213.3±56.2*, △△ IL-10/(pg/mL) 111.5±10.2 41.7±5.9# 58.9±6.8△ 77.1±10.3△△ 127.1±8.5*, △△ TGF-β/(pg/mL) 12.5±1.3 5.1±0.5# 6.7±0.7△ 7.7±0.9△△ 11.5±1.7*, △△ #P<0.01, vs. control group; △P<0.05, △△P<0.01, vs. EAE group; *P<0.05,vs. BMSCs group. 2.4 NBP对BMSCs移植后小鼠脑GFAP、MAP-2和MBP荧光表达量的影响
免疫荧光双标结果显示:在两个BMSCs移植组中,BMSCs+NBP组的GFAP(图4A)、MAP-2(图4B)和MBP(图4C)的表达均较BMSCs组增加(P<0.05)。其中,以MAP-2、MBP的表达增加尤为显著(P<0.01)。表明NBP可能有促进BMSCs向星形胶质细胞、神经元和少突胶质细胞分化的作用。并且,MAP-2和MBP的表达比GFAP的表达提升更为明显。
![]() 图 4 NBP对BMSCs移植后GFAP、MAP-2和MBP荧光表达量的影响Figure 4. Effect of NBP on GFAP, MAP-2 and MBP fluorescence expression after BMSCs transplantationCompared with the group that had BMSCs transplantation alone, the expression of GFAP (A), MAP-2 (B), MBP (C) and the immunofluorescence double labeling(merged with GFP)in the brain of mice significantly increased after NBP was used in combination. White arrows: Immunofluorescence positive cells.* P<0.05; ****P<0.0001; n=10.
图 4 NBP对BMSCs移植后GFAP、MAP-2和MBP荧光表达量的影响Figure 4. Effect of NBP on GFAP, MAP-2 and MBP fluorescence expression after BMSCs transplantationCompared with the group that had BMSCs transplantation alone, the expression of GFAP (A), MAP-2 (B), MBP (C) and the immunofluorescence double labeling(merged with GFP)in the brain of mice significantly increased after NBP was used in combination. White arrows: Immunofluorescence positive cells.* P<0.05; ****P<0.0001; n=10.2.5 各组小鼠脊髓组织NF-κB、PI3K/Akt信号通路及IL-17、Foxp3表达的变化
Western blot显示(图5):与空白对照组相比,EAE组的p-NF-κB/NF-κB比值升高,p-IκBα/IκBα比值稍有升高,差异均有统计学意义(P<0.05);与EAE组相比,NBP组、BMSCs组及BMSCs+NBP组的p-NF-κB/NF-κB比值下降,而p-IκBα/IκBα比值上升,以BMSCs+NBP组最为显著,差异均有统计学意义(P<0.05)。与空白对照组相比,EAE组p-PI3K/PI3K比值下降,差异有统计学意义(P<0.05),而p-Akt/Akt比值无明显变化;与EAE组相比,NBP组、BMSCs组及BMSCs+NBP组的p-PI3K/PI3K及p-Akt/Akt比值均上升,以BMSCs+NBP组最为明显,差异均有统计学意义(P<0.05)。与空白对照组相比,EAE组IL-17/Foxp3比值升高,差异有统计学意义(P<0.05);与EAE组相比,NBP组、BMSCs组及BMSCs+NBP组的IL-17/Foxp3比值降低,尤以BMSCs+NBP组最为明显,差异均有统计学意义(P<0.05)。
![]() 图 5 各组小鼠脊髓组织NF-κB、PI3K/Akt信号通路及IL-17、Foxp3表达的变化Figure 5. Changes of NF-κB and PI3K/Akt signaling pathways and IL-17 and Foxp3 expression in spinal cord of mice in each groupA: Control group; B: EAE group; C: NBP group; D: BMSCs group; E: BMSCs+NBP group. * P<0.05,** P<0.01, *** P<0.001,****P<0.000 1, n=10.
图 5 各组小鼠脊髓组织NF-κB、PI3K/Akt信号通路及IL-17、Foxp3表达的变化Figure 5. Changes of NF-κB and PI3K/Akt signaling pathways and IL-17 and Foxp3 expression in spinal cord of mice in each groupA: Control group; B: EAE group; C: NBP group; D: BMSCs group; E: BMSCs+NBP group. * P<0.05,** P<0.01, *** P<0.001,****P<0.000 1, n=10.3. 讨论
目前认为CD4+ T细胞的分化方向左右着EAE/MS的病情进展。T细胞向Th1和Th17细胞的进一步分化会加剧EAE/MS病情进展,而分化为Th2细胞和Treg细胞,则起到保护作用[9]。因此,EAE/MS的免疫发病机制与Th17和Treg细胞平衡受损息息相关。Th17细胞通过产生标志性细胞因子IL-17、IL-22和包括IL-6在内的其他炎症介质,促进MS炎症和神经退行性病变[10-11]。与Th17细胞相反,Treg细胞主要通过分泌IL-10和TGF-β来抑制免疫应答[12],Treg细胞及其转录因子Foxp3在EAE免疫调节中起负向作用,抑制EAE病理的进展[13]。
NF-κB信号通路的激活可促进MS/EAE中胶质细胞的激活、介导血脑屏障(blood-brain barrier, BBB)的破坏及加速外周免疫细胞进入脑实质,而阻断NF-κB通路会使EAE症状明显缓解[14]。NF-κB通路的激活可显著促进Th17细胞的分化。雷帕霉素与芬戈莫德联合治疗可抑制EAE小鼠Th17细胞的分化和功能,增加脾脏和中枢神经系统的Treg细胞,改善EAE小鼠的症状和脱髓鞘改变[15]。本研究发现:与EAE组比较,NBP和BMSCs均可上调Foxp3和下调IL-17;Th17细胞产生的细胞因子分泌下降,Treg细胞产生的细胞因子量上升。已有学者发现,NBP通过减少Th17并增加Treg细胞的数量来发挥其免疫调节作用[16]。间充质干细胞(mesenchymal stem cells, MSCs)能诱导Th17细胞直接转化为Treg细胞[17]。NBP也可以通过抑制c-Jun末端激酶及NF-κB途径激活来减轻脂多糖诱导的大鼠炎症反应[18]。而BMSCs移植的免疫调节作用也是通过抑制NF-κB通路实现的[19]。本研究中,与空白对照组相比,p-NF-κB/NF-κB、IL-17/Foxp3比值的升高均反映出EAE小鼠体内炎性反应水平的提升;经过治疗后,以上比值均显著下降,尤以联合治疗为明显。p-IκBα/IκBα比值在给予治疗干预后,出现了明显上升,尤以联合治疗最显著。因此,NBP和BMSCs的联用实现了“强强联合”,使两者独自的作用得到了放大。
干细胞的成神经分化可直接促进EAE神经功能的恢复和减轻髓鞘脱失[20-21],然而干细胞分化为神经细胞的比例仍然偏低,治疗效果受到限制。PI3K/Akt通路在神经新生及神经元存活、轴突树突形成和突触间信息传递中发挥重要作用[22]。激活PI3K/Akt通路可促进BMSCs的定向分化。阿托伐他汀可以通过激活PI3K通路恢复神经干细胞活力,促进脑神经再生[23]。丙戊酸钠激活PI3K/Akt通路是诱导神经干细胞分化的必要条件[24]。在大脑中动脉闭塞(middle cerebral artery occlusion, MCAO)大鼠模型,激活PI3K/Akt信号通路可促进神经干细胞的增殖和分化[25]。在体外,NBP通过激活PI3K/Akt通路可减少大鼠BMSCs的凋亡[26]。本研究中,经过治疗后,p-PI3K/PI3K比值均明显回升,说明NBP、BMSCs及BMSCs+NBP治疗均可改善PI3K的抑制,以联合治疗最为明显。与空白对照组相比,EAE组p-Akt/Akt比值无明显变化,说明EAE组的病理改变并未引起Akt的活化。3种治疗均明显导致了p-Akt/Akt比值的升高。进一步分析Western blot的实验结果发现:治疗组对Akt的活化是通过提升p-Akt,而不是改变Akt实现的。总之,BMSCs与NBP联合治疗与单独治疗相比,更有效地激活了PI3K/Akt通路,从而提高EAE小鼠体内BMSCs的成神经分化数量。并且,MAP-2和MBP的表达比GFAP的表达提升更为明显,证明BMSCs更多地向神经元和少突胶质细胞方向分化。因此,NBP可促进BMSCs向神经细胞分化,其机制与激活PI3K/Akt通路有关。
综上所述,NBP和BMSCs联合治疗可有效缓解EAE模型小鼠的症状,减少脱髓鞘病变,且较单独治疗有显著叠加作用,为未来的MS治疗提供了一种新策略,但其机制有待进一步探讨。
* * *
利益冲突 所有作者均声明不存在利益冲突
-
图 1 人骨髓间充质干细胞的形态和表面标记物的表达
Figure 1. Human BMSCs morphology and surface marker expression
BMSCs were attached to a polygonal form. After BMSCs grew to the third generation, their shape became similar to that of fibroblasts (×100) in light microscope (A) and fluorescence microscope (B); C-F: CD29, CD34, CD45 and CD90 surface marker expression was determined by flow cytometry. The result showed that cultured BMSCs expressed CD29 and CD90, while there was no expression of CD34 and CD45 on the surface of BMSCs. R1: Regin 1; RN1: Positive fluorescence regin.
图 3 NBP联合BMSCs移植治疗对EAE小鼠脱髓鞘病变的影响。 LFB染色 ×400
Figure 3. Effect of NBP combined with BMSCs transplantation on demyelinating lesions in EAE mice. LFB staining ×400
A: Control group, no demyelination; B: EAE group, large areas of demyelination (black arrows); C: NBP group, after NBP treatment, compared with the EAE group, there was significantly less demyelination (black arrows); D: BMSCs group, after BMSCs transplantation, the area of demyelination (black arrows) was significantly reduced compared with that of the EAE group; E: BMSCs+NBP group, after combined treatment with NBP and BMSCs, compared with EAE group, demyelination was greatly reduced; compared with NBP and BMSCs alone treatment group, demyelination was further reduced (black arrows). F: Pathological score, n=10, *P<0.05, **P<0.01, *** P<0.001, ****P<0.000 1.
图 4 NBP对BMSCs移植后GFAP、MAP-2和MBP荧光表达量的影响
Figure 4. Effect of NBP on GFAP, MAP-2 and MBP fluorescence expression after BMSCs transplantation
Compared with the group that had BMSCs transplantation alone, the expression of GFAP (A), MAP-2 (B), MBP (C) and the immunofluorescence double labeling(merged with GFP)in the brain of mice significantly increased after NBP was used in combination. White arrows: Immunofluorescence positive cells.* P<0.05; ****P<0.0001; n=10.
图 5 各组小鼠脊髓组织NF-κB、PI3K/Akt信号通路及IL-17、Foxp3表达的变化
Figure 5. Changes of NF-κB and PI3K/Akt signaling pathways and IL-17 and Foxp3 expression in spinal cord of mice in each group
A: Control group; B: EAE group; C: NBP group; D: BMSCs group; E: BMSCs+NBP group. * P<0.05,** P<0.01, *** P<0.001,****P<0.000 1, n=10.
表 1 各组小鼠血清IL-6、IL-17、IL-22、IL-10和TGF-β水平的变化
Table 1 Changes of serum levels of IL-6, IL-17, IL-22, IL-10 and TGF-β in the mice of each group
Index Group Control (n=10) EAE (n=10) NBP (n=10) BMSCs (n=10) BMSCs+NBP (n=10) IL-6/(pg/mL) 91.1±18.5 188.1±35.0# 145.1±17.9△ 132.6±19.6△△ 101.3±21.6*, △△ IL-17/(pg/mL) 50.4±9.1 120.6±10.5# 84.0±6.2△ 74.4±7.7△△ 60.4±8.1*, △△ IL-22/(pg/mL) 223.5±80.6 515.1±58.6# 402.0±77.9△ 339.8±89.0△△ 213.3±56.2*, △△ IL-10/(pg/mL) 111.5±10.2 41.7±5.9# 58.9±6.8△ 77.1±10.3△△ 127.1±8.5*, △△ TGF-β/(pg/mL) 12.5±1.3 5.1±0.5# 6.7±0.7△ 7.7±0.9△△ 11.5±1.7*, △△ #P<0.01, vs. control group; △P<0.05, △△P<0.01, vs. EAE group; *P<0.05,vs. BMSCs group. -
[1] LEMUS H N, WARRINGTON A E, RODRIGUEZ M. Multiple Sclerosis: Mechanisms of disease and strategies for myelin and axonal repair. Neurol Clin, 2018, 36(1): 1−11.
[2] KIPP M, NYAMOYA S, HOCHSTRASSER T, et al. Multiple sclerosis animal models: A clinical and histopathological perspective. Brain Pathol, 2017, 27(2): 123-137.
[3] KIM E A, KIM S Y, YE B R, et al. Anti-inflammatory effect of Apo-9'-fucoxanthinone via inhibition of MAPKs and NF-kB signaling pathway in LPS-stimulated RAW 264.7 macrophages and zebrafish model. Int Immunopharmacol, 2018, 59: 339−346[2020-11-09]. https://doi.org/10.1016/j.intimp.2018.03.034.
[4] HU R, LV W, ZHANG S, et al. Combining miR-23b exposure with mesenchymal stem cell transplantation enhances therapeutic effects on EAE. Immunol Lett, 2021, 229: 18−26[2020-11-09]. https://doi.org/10.1016/j.imlet.2020.11.007.
[5] ZHANG J, BRODIE C, LI Y, et al. Bone marrow stromal cell therapy reduces proNGF and p75 expression in mice with experimental autoimmune encephalomyelitis. J Neurol Sci, 2009, 279(1/2): 30−38[2020-11-09]. https://doi.org/10.1016/j.jns.2008.12.033.
[6] WANG W, WANG T, BAI S, et al. Dl-3-n-butylphthalide attenuates mouse behavioral deficits to chronic social defeat stress by regulating energy metabolism via AKT/CREB signaling pathway. Transl Psychiat, 2020, 10(1): 49[2020-11-09]. https://doi.org/10.1038/s41398-020-0731-z.
[7] LUO R, WANGQIN R, ZHU L, et al. Neuroprotective mechanisms of 3-n-butylphthalide in neurodegenerative diseases. Biomed Rep, 2019, 11(6): 235−240.
[8] CORREA J O, AARESTRUP B J, AARESTRUP F M. Effect of thalidomide and pentoxifylline on experimental autoimmune encephalomyelitis (EAE). Exp Neurol, 2010, 226(1): 15−23 .
[9] CAI Y, SHEN H, QIN C, et al. The spatio-temporal expression profiles of CD4+ T cell differentiation and function-related genes during EAE pathogenesis. Inflammation, 2017, 40(1): 195−204.
[10] BALASA R, BARCUTEAN L, BALASA A, et al. THE action of TH17 cells on blood brain barrier in multiple sclerosis and experimental autoimmune encephalomyelitis. Hum Immunol, 2020, 81(5): 237−243.
[11] SUN D, LUO F, XING J C, et al. 1, 25(OH)2 D3 inhibited Th17 cells differentiation via regulating the NF-kappaB activity and expression of IL-17. Cell Prolif, 2018, 51(5): e12461[2020-11-09]. https://doi.org/10.1111/cpr.12461.
[12] MOAAZ M, YOUSSRY S, ELFATATRY A, et al. Th17/Treg cells imbalance and their related cytokines (IL-17, IL-10 and TGF-beta) in children with autism spectrum disorder. J Neuroimmunol, 2019, 337: 577071[2020-11-09]. https://doi.org/10.1016/j.jneuroim.2019.577071.
[13] WANG N, LIANG S, JIN J, et al. CD226 attenuates Treg suppressive capacity via CTLA-4 and TIGIT during EAE. Immunol Res, 2019, 67(6): 486−496.
[14] CHAO C C, GUTIERREZ-VAZQUEZ C, ROTHHAMMER V, et al. Metabolic control of astrocyte pathogenic activity via cPLA2-MAVS. Cell, 2019, 179(7): 1483−1498 e1422[2020-11-09]. https://doi.org/10.1016/j.cell.2019.11.016.
[15] HOU H, CAO R, QUAN M, et al. Rapamycin and fingolimod modulate Treg/Th17 cells in experimental autoimmune encephalomyelitis by regulating the Akt-mTOR and MAPK/ERK pathways. J Neuroimmunol, 2018, 324: 26−34[2020-11-09]. https://doi.org/10.1016/j.jneuroim.2018.08.012.
[16] 毛国富, 欧阳玉龙, 房树华. 丁苯酞对老年急性脑梗死患者外周血Th17/Treg水平及相关细胞因子表达的影响. 湖北科技学院学报(医学版), 2019, 33(3): 212-215. [17] CHEN Q H, WU F, LIU L, et al. Mesenchymal stem cells regulate the Th17/Treg cell balance partly through hepatocyte growth factor in vitro. Stem Cell Res Ther, 2020, 11(1): 91[2020-11-09]. https://doi.org/10.1186/s13287-020-01612-y.
[18] YANG M, DANG R, XU P, et al. Dl-3-n-Butylphthalide improves lipopolysaccharide-induced depressive-like behavior in rats: Involvement of Nrf2 and NF-kappaB pathways. Psychopharmacology (Berl), 2018, 235(9): 2573−2585.
[19] LOTFY A, ALI N S, ABDELGAWAD M, et al. Mesenchymal stem cells as a treatment for multiple sclerosis: A focus on experimental animal studies. Rev Neurosci, 2020, 31(2): 161−179.
[20] CHEN T, NOTO D, HOSHINO Y, et al. Butyrate suppresses demyelination and enhances remyelination. J Neuroinflamm, 2019, 16(1): 165[2020-11-09]. https://doi.org/10.1186/s12974-019-1552-y.
[21] LI Q, HOUDAYER T, LIU S, et al. Induced neural activity promotes an oligodendroglia regenerative response in the injured spinal cord and improves motor function after spinal cord injury. J Neurotrauma, 2017, 34(24): 3351−3361.
[22] FAN W, LI X, HUANG L, et al. S-oxiracetam ameliorates ischemic stroke induced neuronal apoptosis through up-regulating α7 nAChR and PI3K/Akt/GSK3β signal pathway in rats. NeurochemInt, 2018, 115: 50−60[2020-11-09]. https://doi.org/10.1016/j.neuint.2018.01.008.
[23] CHOI N Y, KIM J Y, HWANG M, et al. Atorvastatin rejuvenates neural stem cells injured by oxygen-glucose deprivation and induces neuronal differentiation through activating the PI3K/Akt and ERK pathways. Molecul Neurobiol, 2019, 56(4): 2964−2977 .
[24] ZHANG X, HE X, LI Q, et al. PI3K/AKT/mTOR signaling mediates valproic acid-induced neuronal differentiation of neural stem cells through epigenetic modifications. Stem Cell Rep, 2017, 8(5): 1256−1269 .
[25] ZHOU Z, DUN L, WEI B, et al. Musk ketone induces neural stem cell proliferation and differentiation in cerebral ischemia via activation of the PI3K/Akt signaling pathway. Neuroscience, 2020, 435: 1−9[2020-11-09]. https://doi.org/10.1016/j.neuroscience.2020.02.031.
[26] SUN B, FENG M, TIAN X, et al. DL-3-n-Butylphthalide protects rat bone marrow stem cells against hydrogen peroxide-induced cell death through antioxidation and activation of PI3K-Akt pathway. Neurosci Lett, 2012, 516(2): 247-252.
-
期刊类型引用(5)
1. 史粮坤,田君明,吴文强,张珊珊. 黄芪糖蛋白的药理作用研究进展. 基层中医药. 2025(01): 110-116 .  百度学术
百度学术
2. 牛珍,吴小杰,杨亮,马芷璇,杨君雄,冯英. 基于广州管圆线虫感染导致Balb/c小鼠大脑脱髓鞘的非靶向代谢组学分析. 中山大学学报(医学科学版). 2025(02): 293-300 .  百度学术
百度学术
3. 李雪,王梦涵,张艳,张晶晶. 鼠神经生长因子联合丙种球蛋白治疗慢性格林巴利综合征临床疗效观察. 实用医院临床杂志. 2024(01): 155-158 .  百度学术
百度学术
4. 潘小龙,应春苗,刘飞祥,张运克. 中药联合干细胞治疗多发性硬化研究进展. 中华中医药学刊. 2023(06): 155-160 .  百度学术
百度学术
5. 刘霞,马金玉. 丁苯酞对慢性吸烟患者脑循环的影响. 中国药物滥用防治杂志. 2022(01): 48-50+54 .  百度学术
百度学术
其他类型引用(1)

 首页
首页


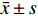

 下载:
下载:







